Novel synthetic method of HIV-1 protease inhibitor atazanavir
A protease inhibitor, HIV-1 technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of high production cost and many synthesis steps, and achieve the effect of simple operation, high purity and good stability
Inactive Publication Date: 2010-12-01
SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
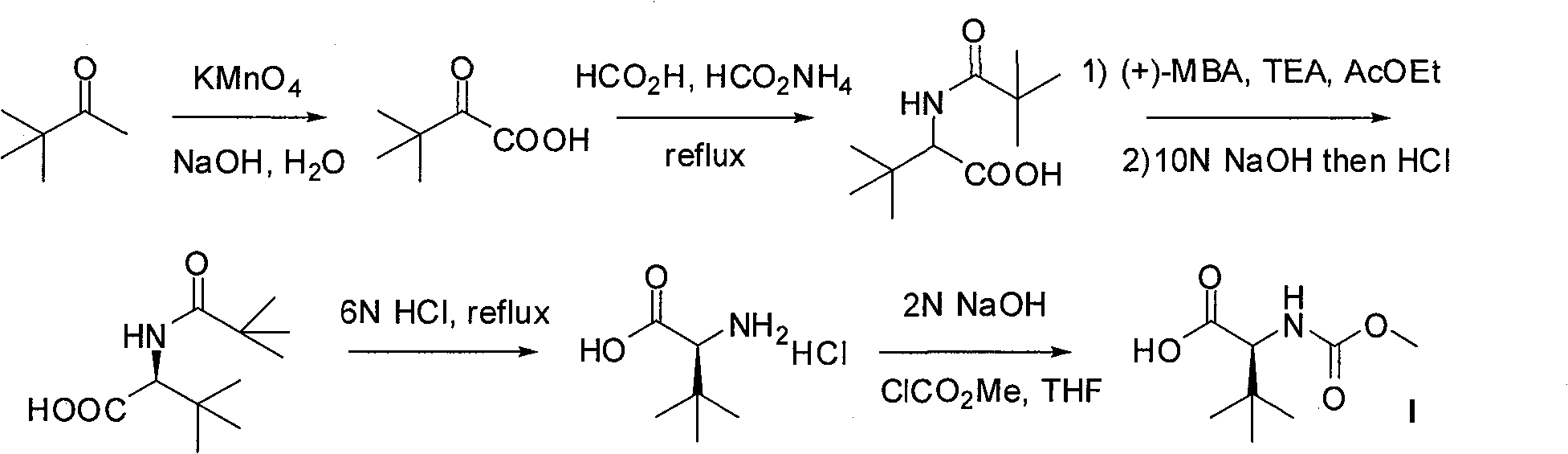
This preparation method is the same as the original route when constructing the basic skeleton of the compound, but the chiral epoxy intermediate is prepared from the more expensive chiral diol through a 4-step reaction through a protecting group strategy. Compared with the original route, this route has improved The optical purity of intermediates, but more synthesis steps and higher production costs
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
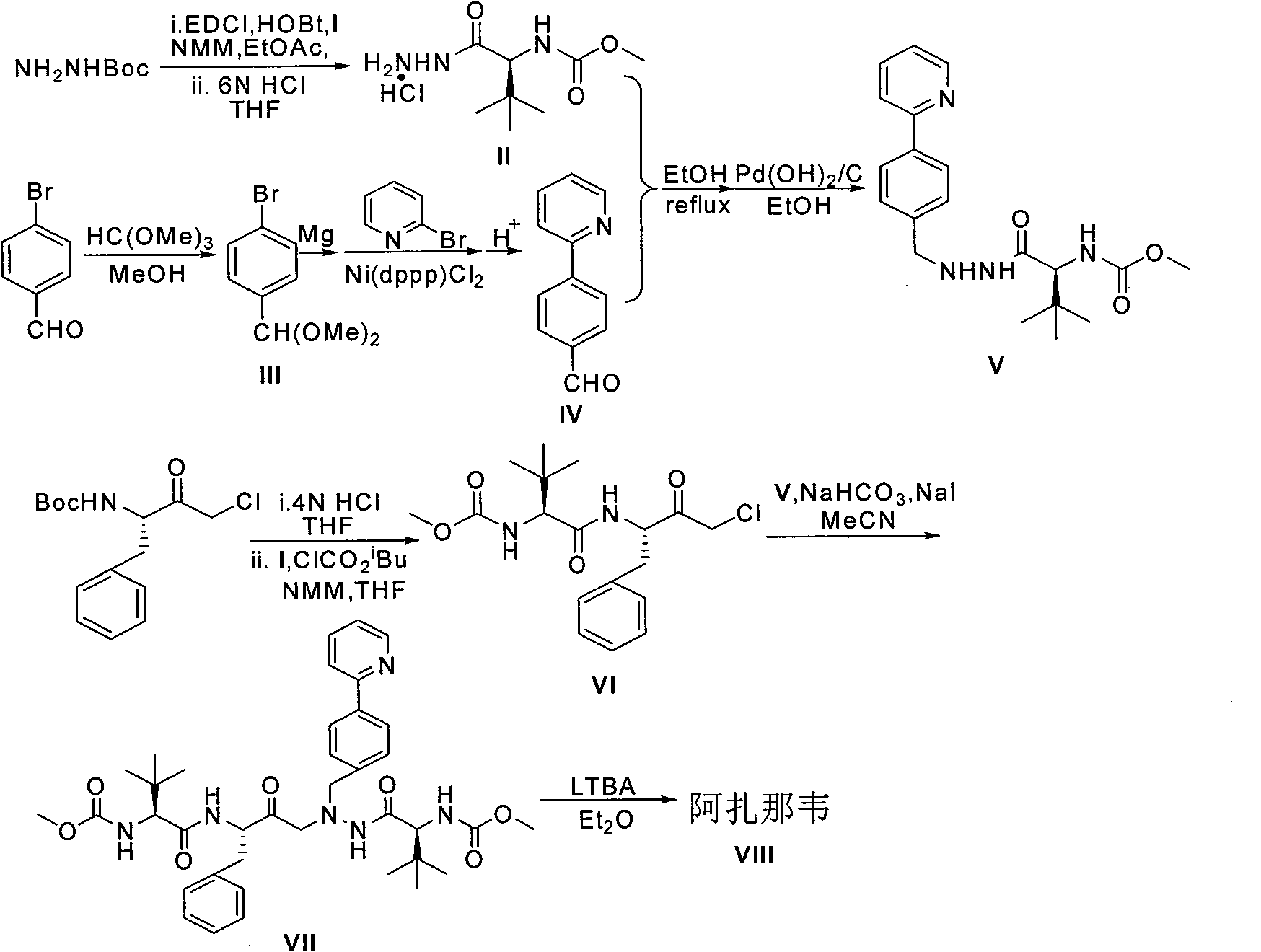
The invention relates to a new synthetic method of an HIV-1 protease inhibitor, Atazanavir, which adopts a convergent-typed synthetic strategy, introduces a construction unit, methoxycarbonyl-tert-lencyl, as an N atom protecting group in the whole early synthetic stage, and takes the diastereomeric selective reduction of aminoketone as the key and final reaction step of the new process. The method comprises the steps that: the compound of formula V, namely, N-1-[N-(methoxycarbonyl)-L-tert-leucine]-N-2-[4-(2-pyridyl)-phenmethyl]hydrazine, and the compound of formula VI, namely, (S)-1-((S)-4-chlorine-3-carbonyl-1-phenyl butane-2-yl-2-amino)-3, 3-dimethyl-1-carbonyl butane-2-yl-methyl carbamate, are treated with nucleophilic substitution reaction to generate the compound of formula VII, namely, 1-[4-(2-pyridyl)phenyl]-5(S)-2, 5-bis{[N-(methoxycarbonyl)-L-tert-leucineyl] amino}-4-carbonyl-6-phenyl-2-azahexane; and the compound of formula VII is treated with reduction reaction to generate the Atazanavir. The invention has the advantages of less process route steps, easily-controlled reaction conditions, simple and convenient operation, low-price and easily-obtained raw material, high product yield, low cost and being suitable for large-scale production.
Description
Synthetic method of HIV-1 protease inhibitor atazanavir technical field The present invention relates to a new protease inhibitor atazanavir (Atazanavir, 1-[4-(Pyridin-2-yl)phenyl]-5(S)-2,5-bis{[N-(methoxycarbonyl)- The synthetic method of L-tert-leucinyl]amino}-4(S)-hydroxy-6-phenyl-2-azahexane). Background technique Human immunodeficiency virus (HIV) is the pathogen that causes acquired immunodeficiency syndrome (AIDS). During the infection cycle of HIV virions, viral proteases are functional enzymes that cleave polyproteins into structural proteins. Therefore, targeting viral protease inhibitors has become an effective method for the treatment of AIDS. Atazanavir is an open-chain azapeptidomimetic compound and a novel protease inhibitor. Compared with other protease inhibitors, atazanavir has two significant advantages. First, it is the only protease inhibitor that is allowed to be taken once a day, which will greatly simplify the dosage course; second, atazanavir h...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D213/53
CPCY02P20/55
Inventor 龙亚秋樊兴宋艳丽
Owner SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com