Preparation method of tulathromycin
A technology of tebramycin and compound, which is applied in the field of preparation of tebramycin and can solve problems such as difficulty in operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
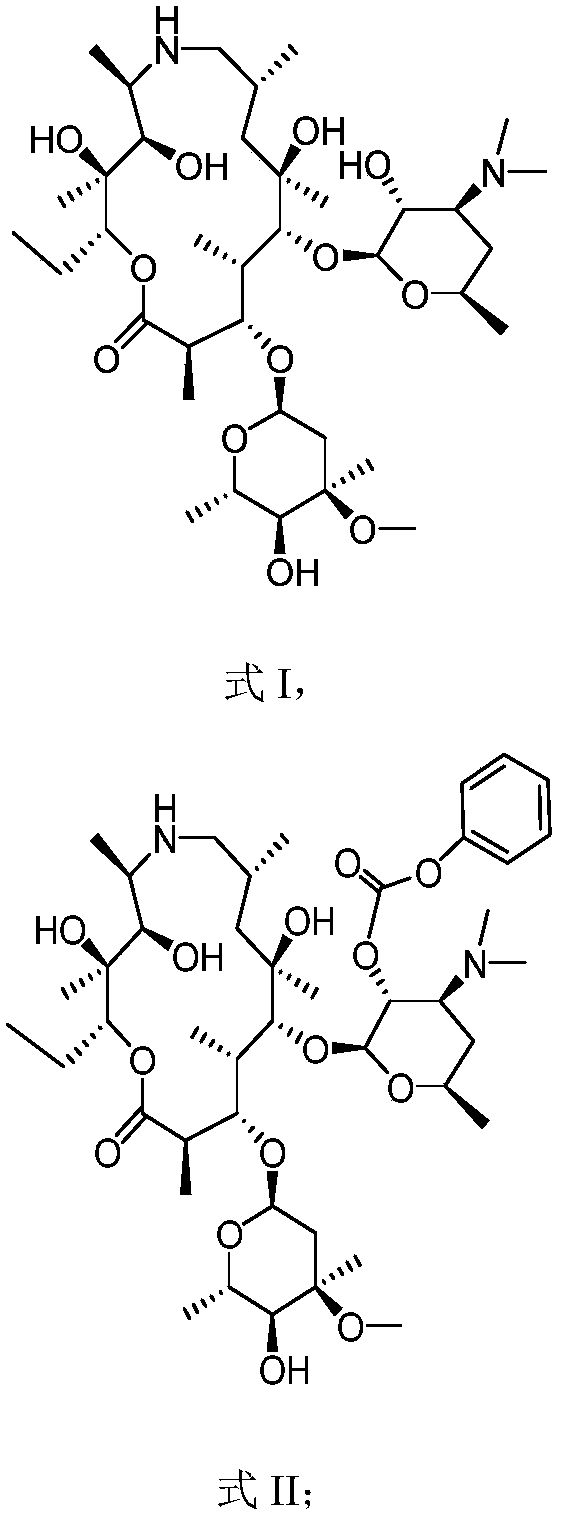
[0045] The preparation of embodiment 1 formula II compound
[0046] Add the compound of formula I (100.00g) and dichloromethane (700.00g) into the reaction flask at room temperature, stir and cool down to -5.0°C, add dropwise a mixture of phenyl chloroformate (22.36g) and dichloromethane (100.00g) The solution was kept at 0°C and stirred for 3 hours. After the reaction was completed, an aqueous solution of sodium carbonate was added, and the liquid was separated to obtain an organic phase, which was concentrated to obtain the compound of formula II, which was directly used in the next step. Yield: 92%.
[0047] MS (ESI) m / z: 855.51 [M+H] + .
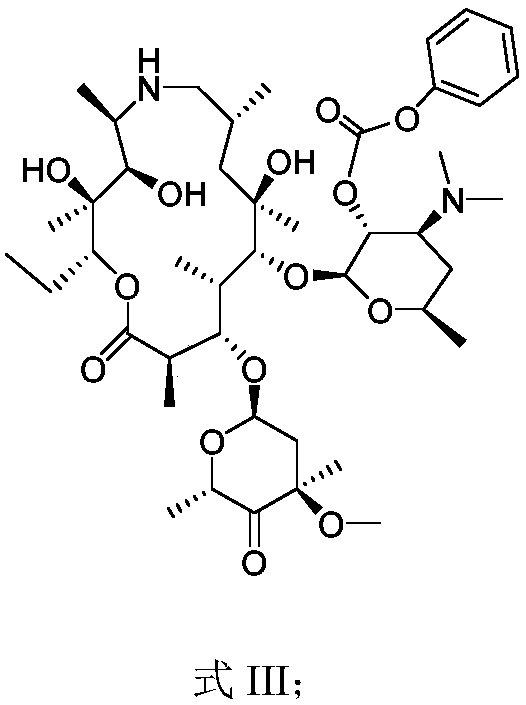
[0048] The preparation of embodiment 2 formula III compound
[0049] Add the compound of formula II in Example 1, o-iodylbenzoic acid (114.32g) and DMSO (300.00g) into the reaction flask at room temperature, add trifluoroacetic acid (23.26g) dropwise, and after the dropwise completion, keep warm at 30°C and stir the reaction. After t...
Embodiment 3
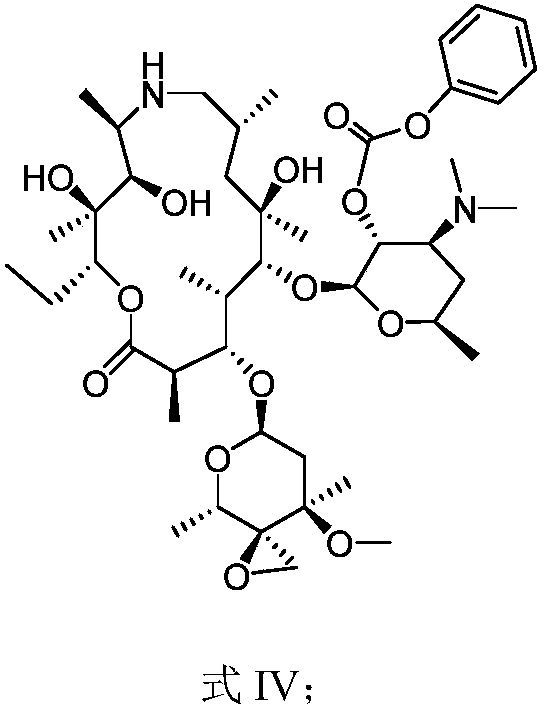
[0051] The preparation of embodiment 3 formula IV compound
[0052] Add Me to the reaction flask at room temperature 3SBr (13.80g) and tetrahydrofuran (100.00g), under the protection of nitrogen, the temperature was lowered to -15°C, potassium tert-butoxide (13.13g) was added, the temperature was lowered to -70°C, and the compound of formula III (25.00g ) in dichloromethane (70.00g), control the internal temperature not to exceed -50°C, add ammonium chloride aqueous solution dropwise to quench the reaction, stir, separate liquids to obtain an organic phase, and concentrate to obtain the compound of formula IV. Yield: 92%.
[0053] MS (ESI) m / z: 867.51 [M+H] + .
Embodiment 4
[0054] The preparation of embodiment 4 formula V compound
[0055] Add the formula IV compound (25.00g) among the embodiment 3 in the reaction bottle, water (100ml) and acetic acid (1.73g), be warming up to 40 ℃ of stirring reaction, reaction is finished, the water ( 50ml) solution, the pH was adjusted to 11.0, and the compound of formula V was obtained by filtration. Yield: 90%.
[0056] MS (ESI) m / z: 747.49 [M+H] +
[0057] 1 H NMR (400MHz, CDCl 3 ), δ: 3.73 (dd, J = 7.4, 117Hz, H-11), 3.30 (s, 12-OH), 2.70 (dq, J = 6.6, 1.7Hz, H-10), 2.49 (dJ = 8.75Hz ,H-9),2.33(s,NMe),2.30(s,NMe2),2.04(t,H-9ax),2.02(m,8-H),1.16(d,J=6.5Hz,10-CH3 ), 1.09(s, 12-CH3), 1.05(d, J=7.6Hz, 4-CH3), 0.94(d, J=6.7Hz, 8-H3), 3.05(s, NH), 5.42(s, CH2), 7.19 (s, 5H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com