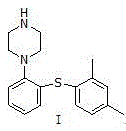
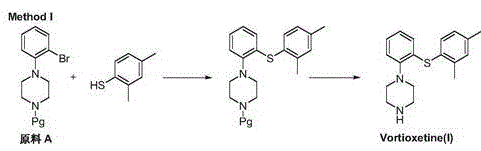
Novel method for preparing vortioxetine
A technology of vortioxetine and compounds, applied in the field of new process synthesis, can solve the problems of unfavorable industrialization, rare raw materials, cumbersome steps, etc., and achieve the effect of quality, environmental protection, economy, easy access to raw materials, and high quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
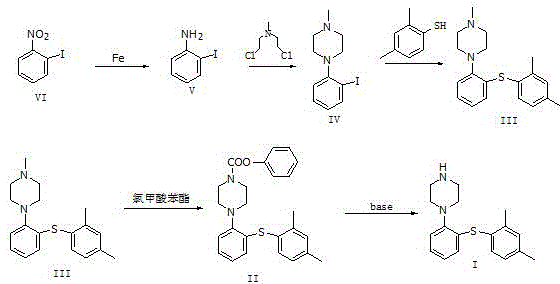
[0032] Add 5.0 g of o-nitroiodobenzene (compound VI) and 15.0 g of reduced iron powder into the reaction flask, then add 25 ml of DMF to dissolve, stir until completely dissolved, add 50 ml of concentrated hydrochloric acid dropwise, and heat to reflux after dropping. After 2 hours, the system was suction-filtered while it was hot, and the filtrate was spin-dried to obtain 4.36 g of a light yellow solid, namely o-aminoiodobenzene (compound V), with a yield of 99.2%.
Embodiment 2
[0034] Dissolve 4.36 g of o-aminoiodobenzene (compound V) in 43.6 mL of toluene, add 9.3 g of N,N-bis-(2-chloroethyl)methylamine and 8.72 g of sodium carbonate, stir and raise the temperature to reflux, and monitor by TLC. After the reaction was complete, the temperature was lowered, washed with water and saturated saline, separated, and the organic phase was spin-dried to obtain an oily substance, stirred with n-hexane to precipitate a solid, filtered with suction, and the filter cake was dried to obtain 5.13g, which was 1-(2-iodophenyl) -4-methylpiperazine (compound IV), yield 84.9%.
Embodiment 3
[0036] Dissolve 5.13g of 1-(2-iodophenyl)-4-methylpiperazine (compound IV) in 25mL of DMF, add 1.54g of potassium carbonate, and heat to 55-60°C. Add 2,4-dimethylthiophenol to the system, and react at a temperature of 80-90°C for 4 hours, and monitor the disappearance of the raw material point by TLC. After the reaction was completed, 75 mL of deionized water was added to the system. During the cooling and stirring process, an off-white solid gradually precipitated out. After cooling down to room temperature, it was suction-filtered, the filter cake was washed twice with methanol, and dried to obtain 4.22 g of an off-white solid, which was 1-[2 -(2,4-Dimethylphenylsulfanyl)-phenyl]methylpiperazine (compound III), yield 85.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com