Novel crystal form of regorafenib
A technology of regorafenib and crystal form, applied in organic chemistry and other directions, can solve the problems of long production cycle, poor stability, high hygroscopicity, etc., and achieve the effect of simple preparation process and good crystal form stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 Preparation of D crystal form
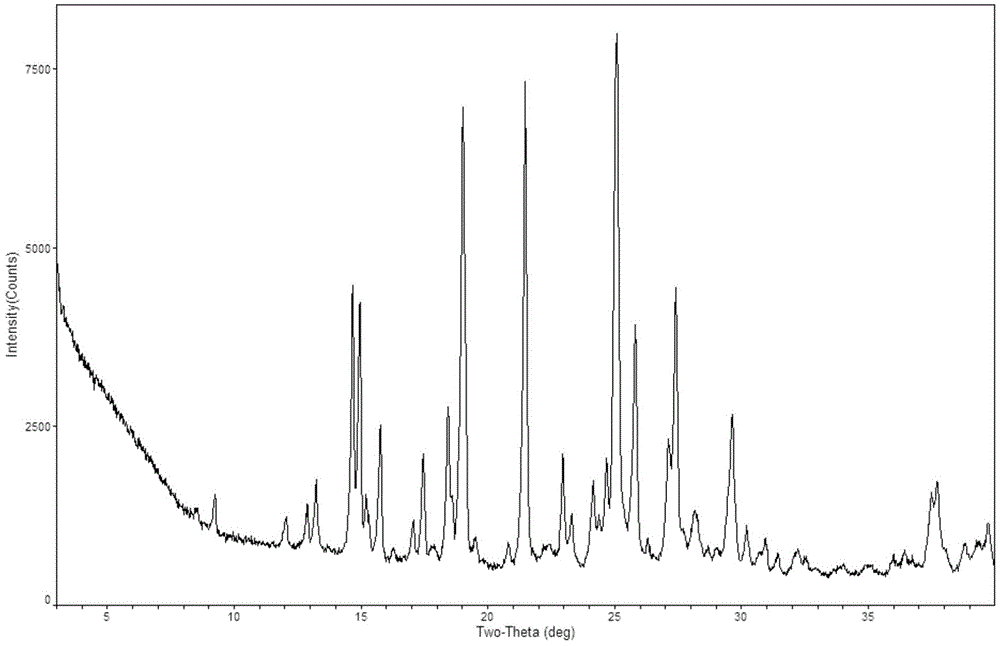
[0035] The acetone solution (50 mL) of formula III (4 g) was dropped into the acetone solution (35 mL) of formula II (3.7 g) at a temperature of 10°C. After dripping, stirred for 24h, solid precipitated out. Filtered, rinsed with acetone, sucked dry, and dried under reduced pressure at 20°C to obtain 5.0 g of a white solid, which was detected by XPRD as crystal form D of regorafenib. HPLC: 99.6%. MS (ESI): m / z=483 (M+H + ).
Embodiment 2
[0036] Embodiment 2 Preparation of D crystal form
[0037] At 20°C, 50 mL of a mixed solution of methylene chloride and acetone of formula III (4g) (dichloromethane: acetone=1:4) was dropped into methylene chloride solution (35 mL) of formula II (3.7 g) . After dripping, stirred for 24h, solid precipitated out. Filter, rinse with acetone, suck dry, and dry under reduced pressure at 20°C to obtain 5.5 g of white crystalline form D of regorafenib. HPLC: 99.5%.
Embodiment 3
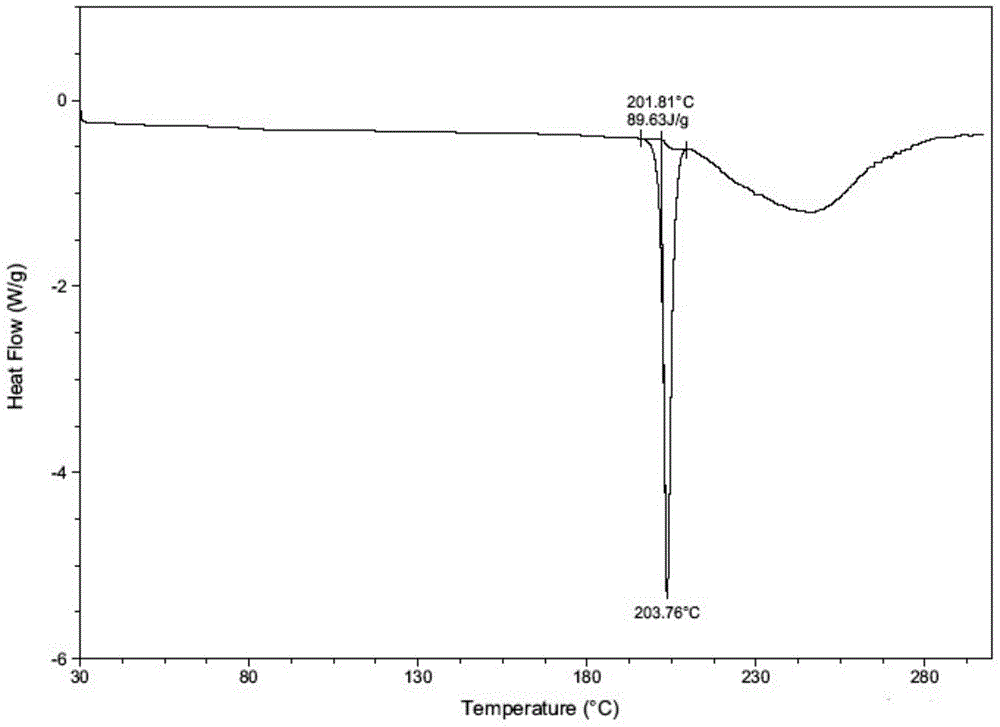
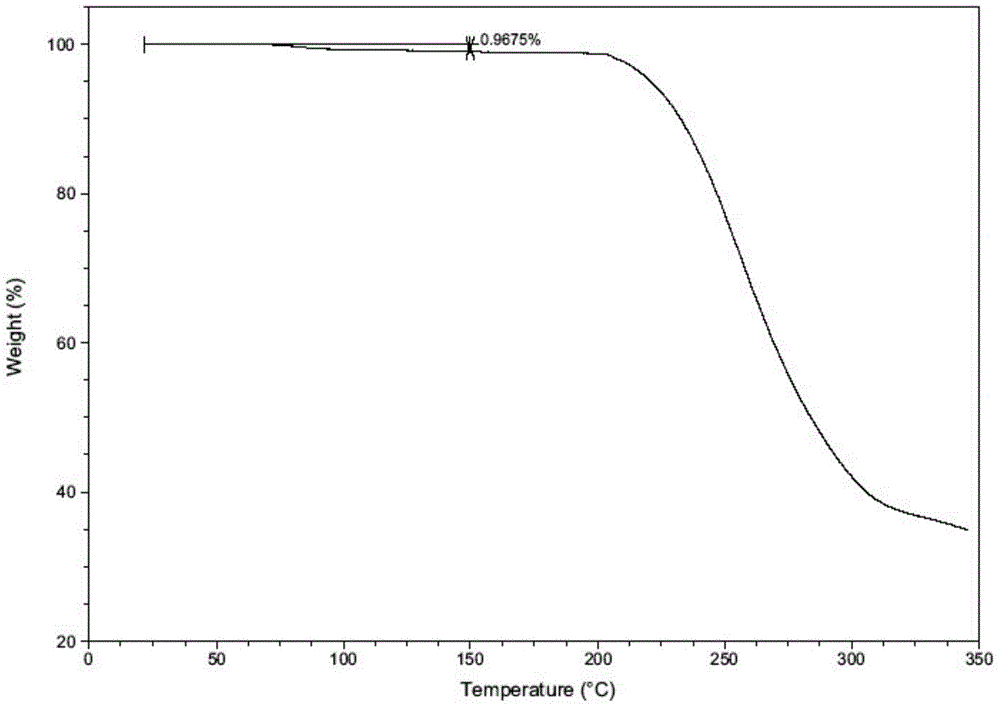
[0038] Example 3 Determination of hygroscopicity and stability
[0039] Test method: refer to the general example XIX C of the second part of the Chinese Pharmacopoeia 2010 edition, weigh the test product that has been ground into a fine powder in a weighing bottle, spread it into a thin layer with a thickness of about 5mm, place it in an airtight container, and place it in a high-humidity (25°C temperature and 90% humidity), high temperature (60°C) and light (4500Lx) conditions, samples were taken on the 5th and 10th days respectively, and the weight was accurately weighed to determine the related substances and contents of the samples, and to determine the X-ray diffraction Determine the crystal form of the sample, and the results are shown in the following table.
[0040]
[0041] 0 days
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com