Novel regorafenib anti-tumor platinum (II) complex and preparation method and application thereof
An anti-tumor and complex technology, applied in the field of novel regorafenib anti-tumor platinum complex and its preparation, to achieve the effect of superior in vitro anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 1.0 mmol ligand RFB and 1.0 mmol cis-PtCl in a 50 mL autoclave 2 (DMSO) 2 , add 25.0mL of anhydrous methanol and 1.0mL of distilled water, heat at 55°C for 36 hours, cool down to room temperature, a large amount of yellow product precipitates, filter with suction, wash the yellow product with methanol, acetone and ether in turn, 45 After vacuum drying at °C, a yellow product was finally obtained with a yield of 95.0%.
[0027] The resulting yellow product is identified:
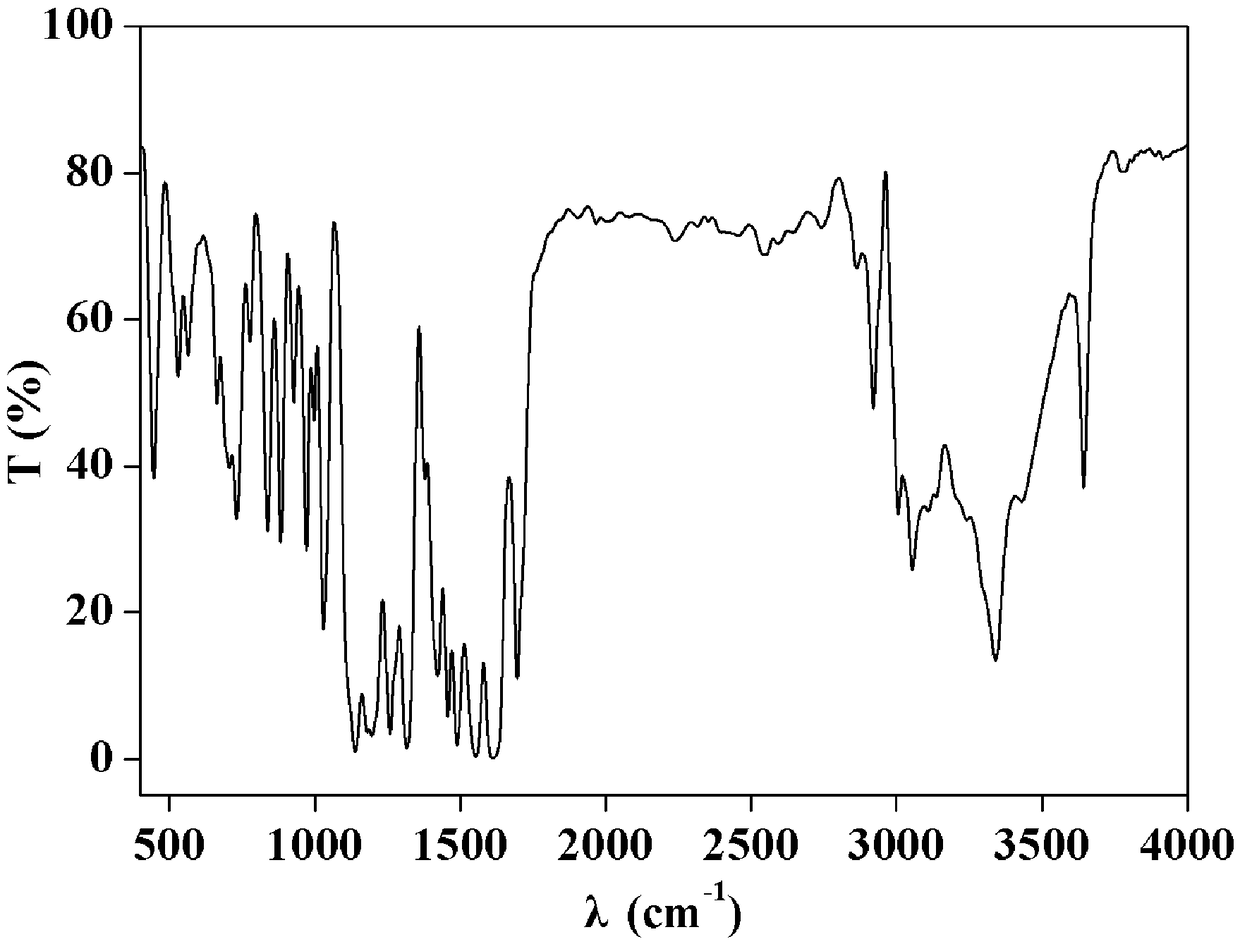
[0028] (1) Infrared spectrum, its spectrogram is as follows figure 1 shown.
[0029] IR(KBr):3644,3341,3054,3006,2921,1696,1614,1490,1458,1423,1317,1197,1159,1140,1031,971,884,838,732,701,663,565,531,446cm -1 .
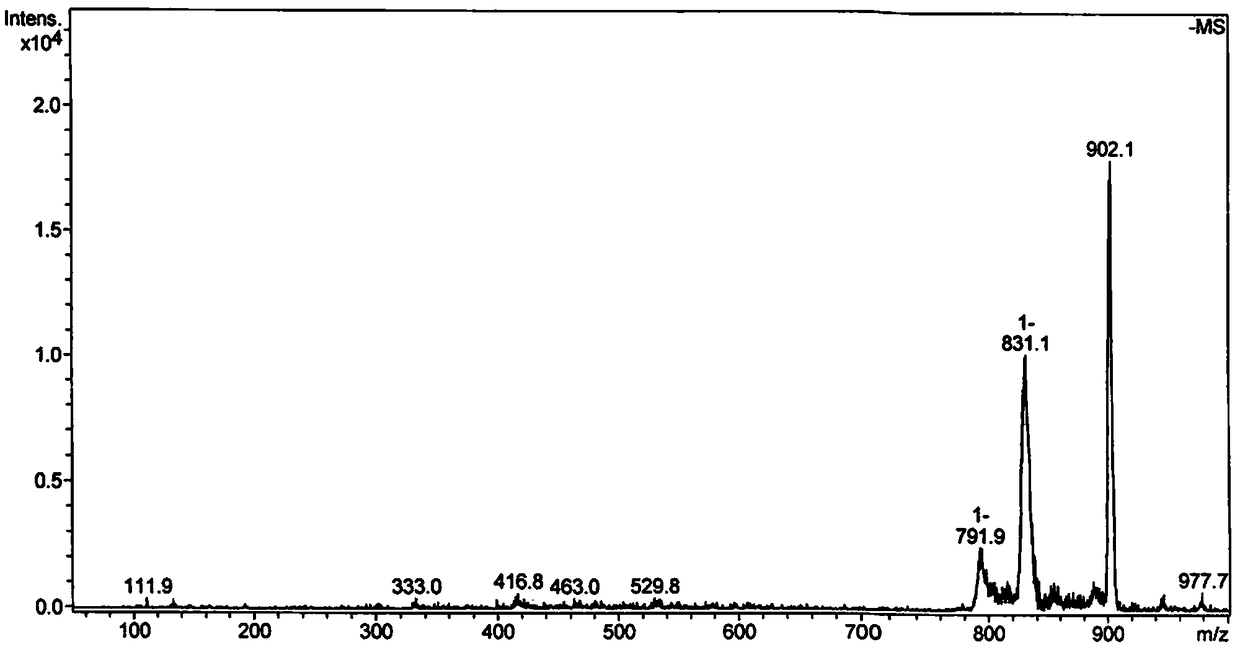
[0030] (2) Electrospray mass spectrometry, its spectrogram is as figure 2 shown.
[0031] ESI-MS m / z:902.1[M-H+DMSO] - , where M is its molecular weight.
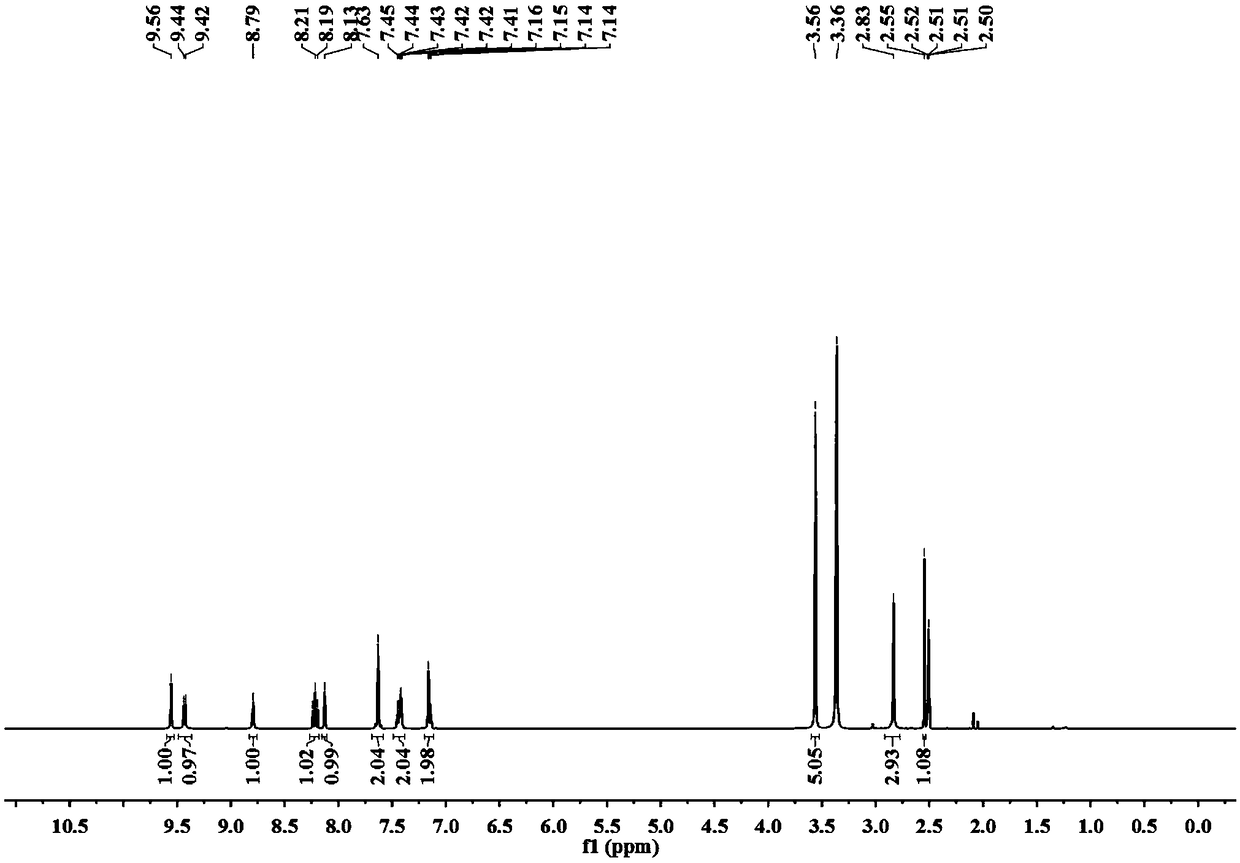
[0032] (3) Proton NMR spectrum, such as image 3 shown.
[0033] 1 H NMR (400MHz, DMSO-d 6 )δ9.56(s,1H),9.43(d,J=6.7Hz,1...
Embodiment 2
[0042] Add ligand 1.0mmol RFB and 5.0mmol cis-PtCl in a 50mL autoclave2 (DMSO) 2 , add 35.0 mL of absolute ethanol and 10.0 mL of distilled water, heat at 80°C for 48 hours, cool down to room temperature, a large amount of yellow product precipitates, filter with suction, wash the yellow product with methanol, acetone and diethyl ether successively, 45 After vacuum drying at °C, complex 1 was finally obtained with a yield of 80.0%.
Embodiment 3
[0044] Add ligand 5.0mmol RFB and 1.0mmol cis-PtCl in a 50mL autoclave 2 (DMSO) 2 , add 5.0mL of acetonitrile and 30.0mL of distilled water, heat at 45°C for 8 hours, cool down to room temperature, a large amount of yellow product precipitates, filter with suction, wash the yellow product with methanol, acetone and diethyl ether in turn, at 45°C After vacuum drying, complex 1 was finally obtained with a yield of 85.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com