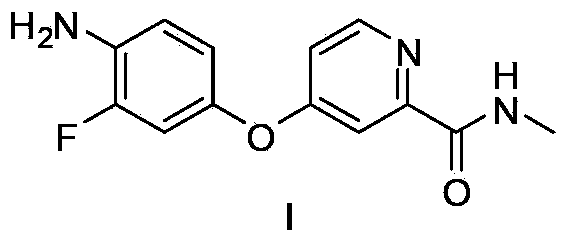
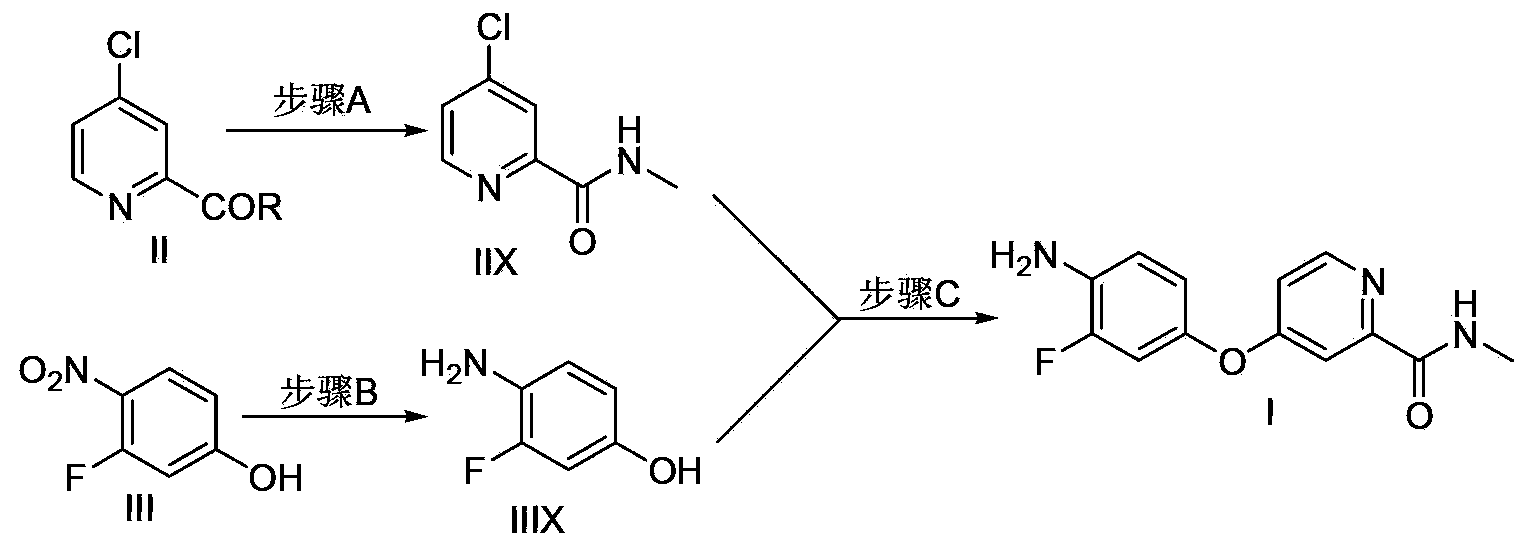
Method for preparing regorafenib intermediate
A technology for regorafenib and intermediates, applied in the field of preparing regorafenib intermediate I, can solve the problems of inconsistent solvent, unfavorable recovery, complicated post-processing and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0014] Example 1 Use tetrahydrofuran as a solvent to synthesize repagliptin intermediate I without separation in each step.
[0015] method 1
[0016] Step A: Add 4-chloropyridine-2-carboxylic acid methyl ester hydrochloride (10.4g, 50mmol) and 40mL THF to a four-neck round bottom flask, cool down to 0°C, and add 2M methylamine in tetrahydrofuran dropwise to the system Solution (150mL, 300mmol), keep the temperature of the system not exceeding 10°C, after the dropwise addition is completed, the system is kept at 5-10°C for 6 hours, filtered through silica gel, rinsed with 50mL tetrahydrofuran on the silica gel, combined with the organic phase, directly used for Step C operation. Organic phase is through HPLC content analysis, obtains product compound IIX8.4g, external standard yield 98%.
[0017] HPLC ES / MS m / z:171((M+H) + )
[0018] Step B: Add 3-fluoro-4-nitrophenol (7.9g, 50mmol), 10%Pd / C (0.4g, 5wt%) and 20mL THF into the autoclave, the system is under 0.3Mpa hydrogen...
example 2
[0027] Example 2 Use toluene as a solvent to synthesize repagliptin intermediate I without separation in each step.
[0028] Step A: Add 4-chloropyridine-2-carboxylic acid methyl ester hydrochloride (10.4g, 50mmol) and 40mL THF to a four-neck round bottom flask, cool down to 0°C, and add 2M methylamine in tetrahydrofuran dropwise to the system solution (50mL, 100mmol), keep the temperature of the system not exceeding 10°C, after the dropwise addition is completed, the system is kept at 5-10°C for 6 hours, filtered through silica gel, rinsed with 50mL tetrahydrofuran on the silica gel, combined with the organic phase, directly used for Step C operation. The organic phase was analyzed by HPLC to obtain 7.5 g of the product compound IIX, with an external standard yield of 87%.
[0029] Step B: Add 3-fluoro-4-nitrophenol (7.9g, 50mmol), 20% Pd / C (0.4g, 5wt%) and 20mL toluene into the autoclave, drop two drops of formic acid, the system is at 0.2MPa Under the hydrogen pressure of...
example 3
[0032] Example 3 Use 1,4-dioxane as a solvent to synthesize repagliptin intermediate I without separation in each step.
[0033] Step A: Add 4-chloropyridine-2-carboxylic acid methyl ester hydrochloride (10.4g, 50mmol) and 40mL of 1,4-dioxane into a four-neck round bottom flask, cool down to 5°C, add dropwise 2M tetrahydrofuran solution of methylamine (150mL, 300mmol), keep the temperature of the system not exceeding 10°C, after the dropwise addition, keep the system at 5-10°C for 12h, filter through silica gel, rinse the silica gel with 50mL THF, combine organic After phase, directly used for step C operation. The organic phase was analyzed by HPLC to obtain 8.2 g of the product compound IIX, with an external standard yield of 96%.
[0034] Step B: Add 3-fluoro-4-nitrophenol (7.9g, 50mmol), 1% Pd / C (1.6g, 20wt%) and 20mL 1,4-dioxane into the autoclave, the system is at 0.1Mpa Under the hydrogen pressure of 30 DEG C, react 12h. After the reaction is completed, filter, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com