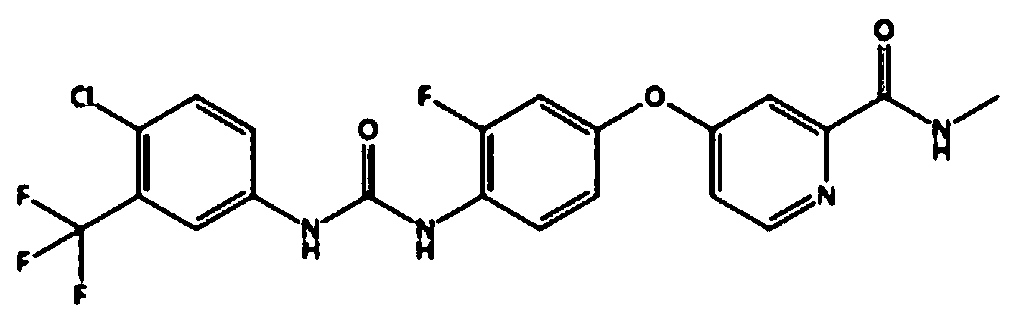
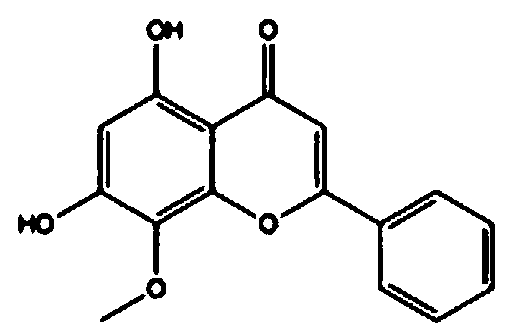
Regorafenib and wogonin eutectic and application thereof
A technology of wogonin and regorafenib, applied in the field of medicine, can solve the problems of low oral bioavailability, influence of absorption and treatment effect, etc., and achieve the effects of improving bioavailability, increasing solubility, and reducing tumor volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
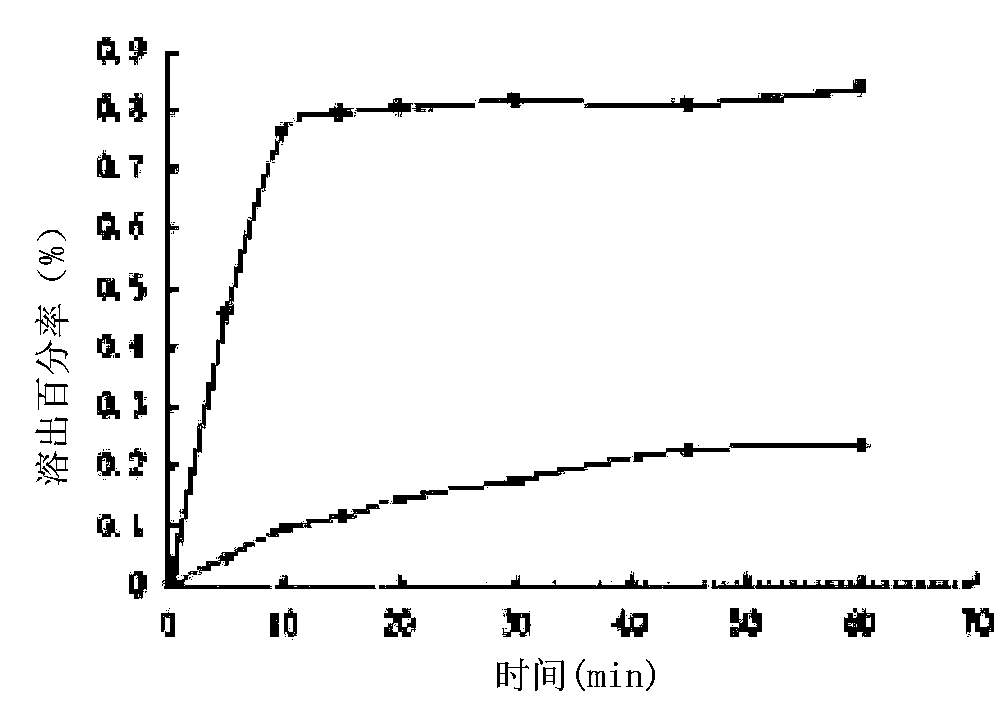
[0033] Dissolve about 173 mg of regorafenib and about 96 mg of wogonin (molar ratio 1:1) in 2-3 mL of acetonitrile:methanol 2:1 (V / V) solvent system, heat to 50°C, and use pore size Filter through a 0.22 μm microporous membrane, then mix the two solutions, let stand at room temperature to volatilize for 24 hours, then wash and filter, and vacuum dry at room temperature for 24 hours to obtain 167 mg of white crystalline solid with a yield of about 62.1%.
Embodiment 2
[0035] Dissolve about 346 mg of regorafenib and about 96 mg of wogonin (molar ratio 2:1) in 2-3 mL of acetonitrile:ethanol 2:1 (V / V) solvent system, heat to 50°C, and use pore size Filter through a 0.22 μm microporous membrane, then mix the two solutions, let stand at room temperature to volatilize for 24 hours, then wash and filter, and vacuum dry at room temperature for 24 hours to obtain 223 mg of white crystalline solid with a yield of about 50.4%.
Embodiment 3
[0037] Dissolve about 173 mg of regorafenib and about 190 mg of wogonin (molar ratio 1:2) in 2-3 mL of acetonitrile:ethyl acetate (2:1) solvent system, heat to 50°C, and use Filter through a 0.22 μm microporous membrane, then mix the two solutions, let stand at room temperature to volatilize for 24 hours, then wash and filter, and vacuum dry at room temperature for 24 hours to obtain 186 mg of white crystalline solid with a yield of about 51.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com