Regorafenib tablet pharmaceutical composition and preparation method thereof
A composition and solvate technology, applied in the field of new anti-tumor drug compositions, can solve the problems of excessive blood vessel proliferation, blindness and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Example 1: Preparation of pharmaceutical composition in tablet form
[0149] Plain Tablet Prescription (per tablet):
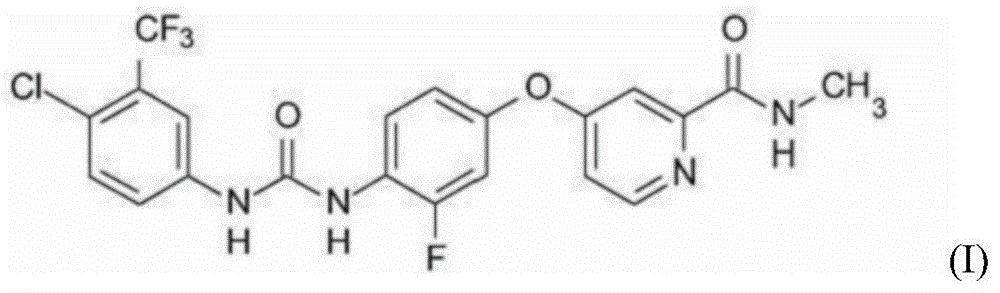
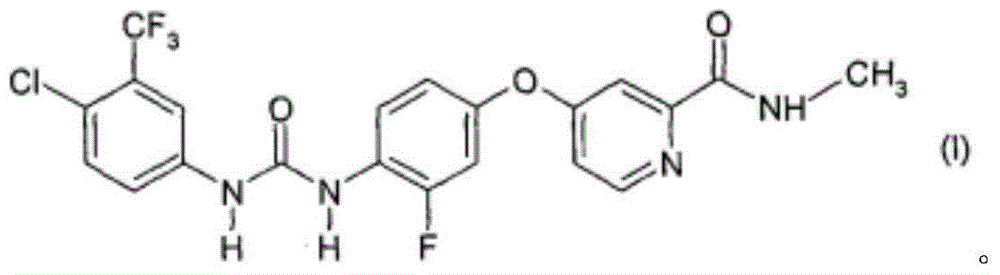
[0150] The monohydrate of the compound of formula I: 40 mg (calculated as anhydrous substance),
[0151] Polyvinylpyrrolidone (k25): 160 mg,
[0152] Croscarmellose sodium (adding part of disintegrant): 100mg,
[0153] Microcrystalline cellulose: 100mg;
[0154] Croscarmellose sodium (additional part of disintegrant): 54mg,
[0155] Colloidal silicon dioxide (anhydrous grade, that is, colloidal silicon dioxide, also known as colloidal anhydrous silica): 2.4mg,
[0156] Magnesium stearate: 3.6 mg.
[0157] Preparation method:
[0158] (1) (a) The active drug of prescription quantity and two kinds of solid materials of polyvinylpyrrolidone are dissolved in solvent ethyl acetate-ethanol mixed solution (ethyl acetate: ethanol=1:4, solid material and solvent weight ratio are 1: 3) to make a drug-containing solution; (b) at a temperature of 60-70...
Embodiment 2
[0162] Embodiment 2: Preparation of pharmaceutical composition in tablet form
[0163] Plain Tablet Prescription (per tablet):
[0164] The monohydrate of the compound of formula I: 40 mg (calculated as anhydrous substance),
[0165] Polyvinylpyrrolidone (k30): 100 mg,
[0166] Croscarmellose sodium (adding part of disintegrant): 150mg,
[0167] Microcrystalline cellulose: 50mg;
[0168] Croscarmellose sodium (additional part of disintegrant): 50mg,
[0169] Colloidal silicon dioxide: 2mg,
[0170] Magnesium stearate: 3 mg.
[0171] Preparation method:
[0172] (1) (a) The active drug of prescription quantity and two kinds of solid materials of polyvinylpyrrolidone are dissolved in solvent ethyl acetate-ethanol mixed solution (ethyl acetate: ethanol=1: 2, solid material and solvent weight ratio are 1: 2) to make a drug-containing solution; (b) at a temperature of 60-70 ° C, use a fluidized bed vacuum granulator to spray the solution into a powder bed of diluent an...
Embodiment 3
[0176] Embodiment 3: Preparation of pharmaceutical composition in tablet form
[0177] Plain Tablet Prescription (per tablet):
[0178] The monohydrate of the compound of formula I: 40 mg (calculated as anhydrous substance),
[0179] Polyvinylpyrrolidone (k15): 200 mg,
[0180] Croscarmellose sodium (adding part of disintegrant): 40mg,
[0181] Microcrystalline cellulose: 200mg;
[0182] Croscarmellose sodium (additional part of disintegrant): 10mg,
[0183] Colloidal silicon dioxide: 2mg,
[0184] Magnesium stearate: 3 mg.
[0185] Preparation method:
[0186] (1) (a) The active drug of prescription quantity and two kinds of solid materials of polyvinylpyrrolidone are dissolved in solvent ethyl acetate-ethanol mixed solution (ethyl acetate: ethanol=1: 6, solid material and solvent weight ratio are 1: 4) to make a drug-containing solution; (b) at a temperature of 60-70°C, use a fluidized bed vacuum granulator to spray the solution into a powder bed of a diluent and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com