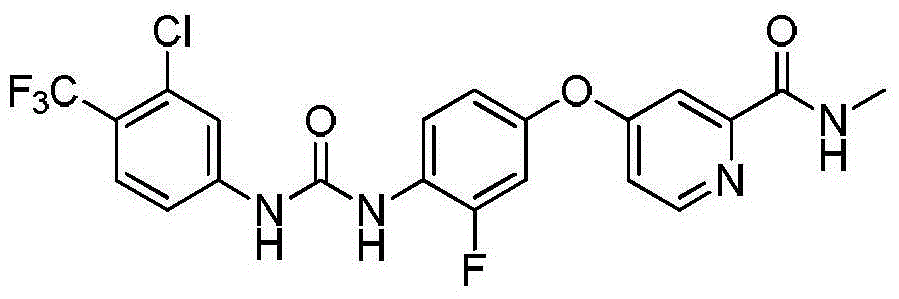
Regorafenib synthesis method by one kettle way
A technology of regorafenib and condensation reaction, which is applied in the field of one-pot synthesis of regorafenib, can solve the problems of unsuitability for industrial production and high production cost, achieve good production and practical value, low production cost, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
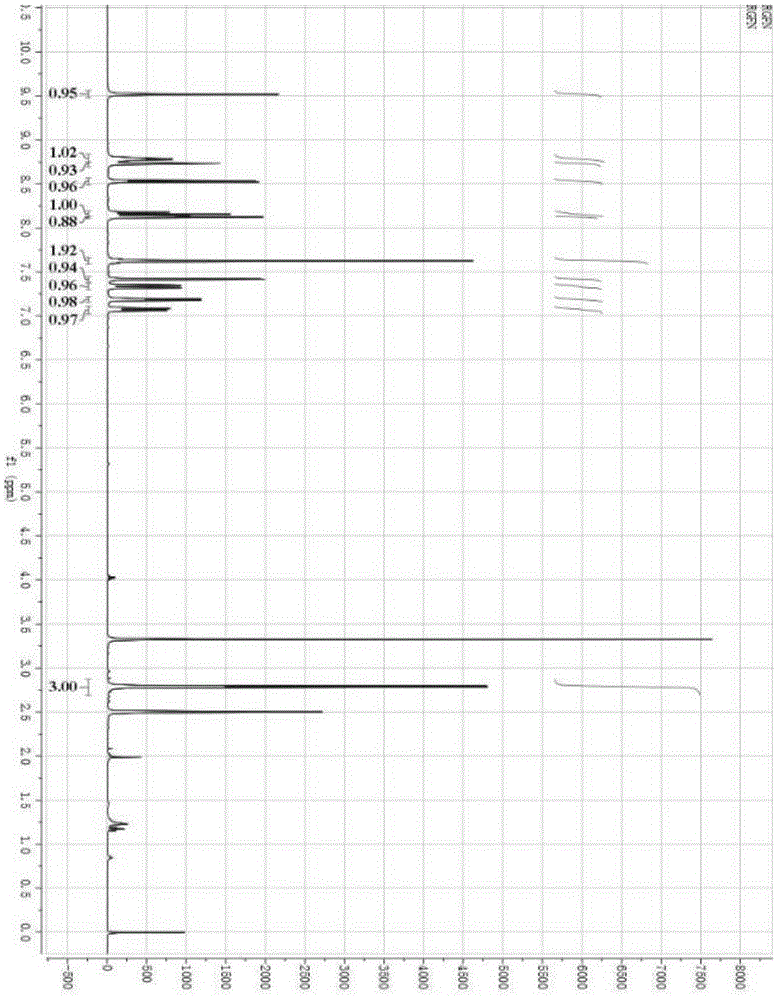
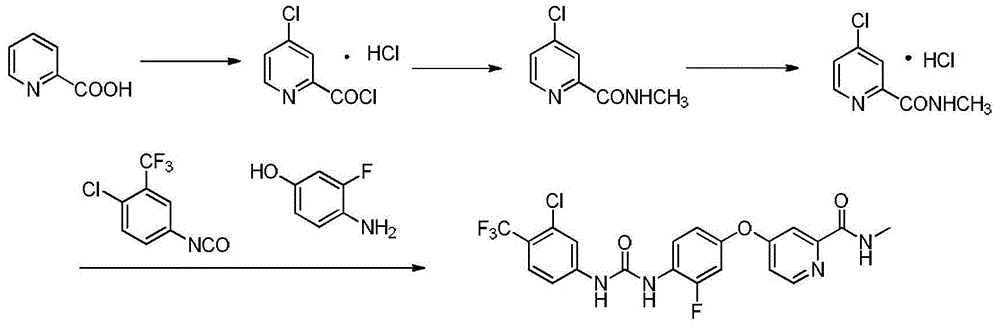
[0024] Add 3-fluoro-4-aminophenol (50g, 225.67mmol), 4-chloro-3-trifluoromethylphenyl isocyanate (31.55g, 248.24mmol) and N,N-dimethyl Formamide. The reaction was stirred at 20°C for 10 minutes. After the reaction, sodium hydroxide (36.1g, 902.67mmol) was added to the reaction solution, stirred for 30min, 4-chloro-N-methyl-2-pyridinecarboxamide (42.35g, 248.24mmol) was added, and the temperature was raised to 130°C for reaction 4h, after the reaction is over, stop heating, cool to room temperature, pour the reaction solution into 1000ml of water, stir in an ice bath for 30min, a large amount of solids are formed, filter with suction, collect the filter cake, recrystallize with ethyl acetate:petroleum ether after drying Off-white solid regorafenib 54.3g, yield 49.83%. The H NMR spectrum of the target product is as figure 1 Shown: 1 H NMR (400MHz, DMSO) δ: 9.52(s, 1H, NHCO), 8.79(d, J=4.8Hz, 1H, CH), 8.74(d, J=1.4Hz, 1H, NHCO), 8.53(d, J=5.6Hz, 1H, CH), 8.16(t, J=9.1Hz, 1H,...
Embodiment 2
[0027] 3-Fluoro-4-aminophenol (50 g, 225.67 mmol), 4-chloro-3-trifluoromethylphenylisocyanate (34.42 g, 270.8 mmol) and dimethyl sulfoxide were added to the reactor. Stir the reaction at 0°C for 20 min. After the reaction, sodium hydroxide (36.1g, 902.67mmol) was added to the reaction solution, stirred for 30min, 4-chloro-N-methyl-2-pyridinecarboxamide (46.20g, 270.80mmol) was added, and the temperature was raised to 80°C for reaction 6h, after the reaction is over, stop heating, cool to room temperature, pour the reaction solution into 1000ml of water, stir in an ice bath for 30min, a large amount of solids are formed, filter with suction, collect the filter cake, recrystallize with ethyl acetate:petroleum ether after drying Off-white solid regorafenib 50.2g, yield 46.07%.
[0028] Melting point: 208.9~210.6℃
Embodiment 3
[0030] Add 3-fluoro-4-aminophenol (50g, 225.67mmol), 4-chloro-3-trifluoromethylphenyl isocyanate (37.29g, 293.37mmol) and N,N-dimethyl Formamide. Stir the reaction at -20°C for 30 minutes. After the reaction, sodium hydroxide (45.13g, 1.13mol) was added to the reaction solution, the reaction was stirred for 30min, 4-chloro-N-methyl-2-pyridinecarboxamide hydrochloride (56.07g, 270.8mmol) was added, and the temperature was raised React at 100°C for 5 hours. After the reaction, stop heating and cool to room temperature. Pour the reaction solution into 1000ml of water and stir for 30 minutes. A large amount of solids are formed. Filter with suction to collect the filter cake. After drying, use ethyl acetate:petroleum Ether recrystallized off-white solid regorafenib 51.0g, yield 46.81%.
[0031] Melting point: 207.9~209.1℃
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com