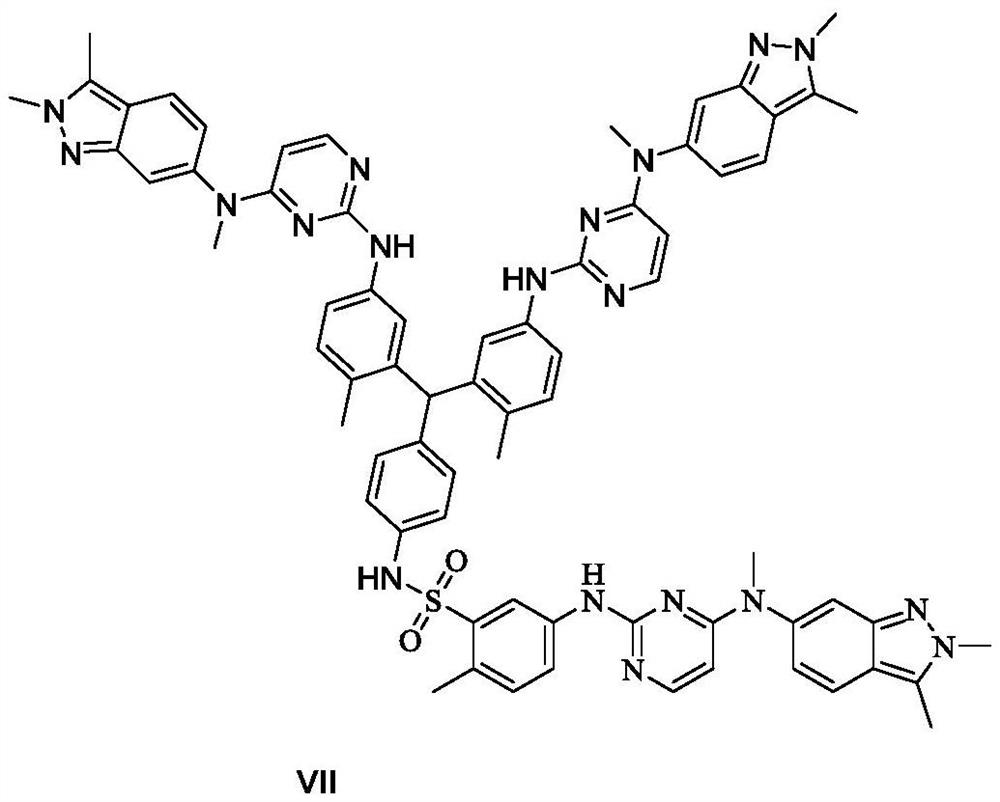
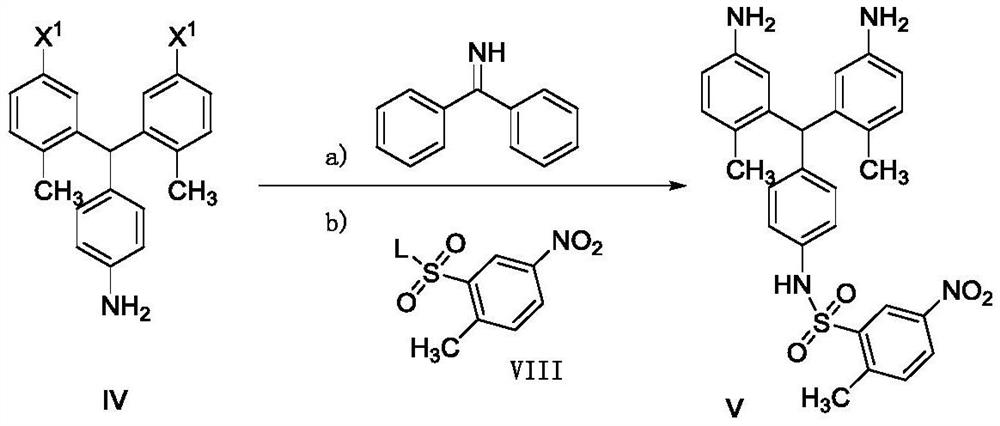
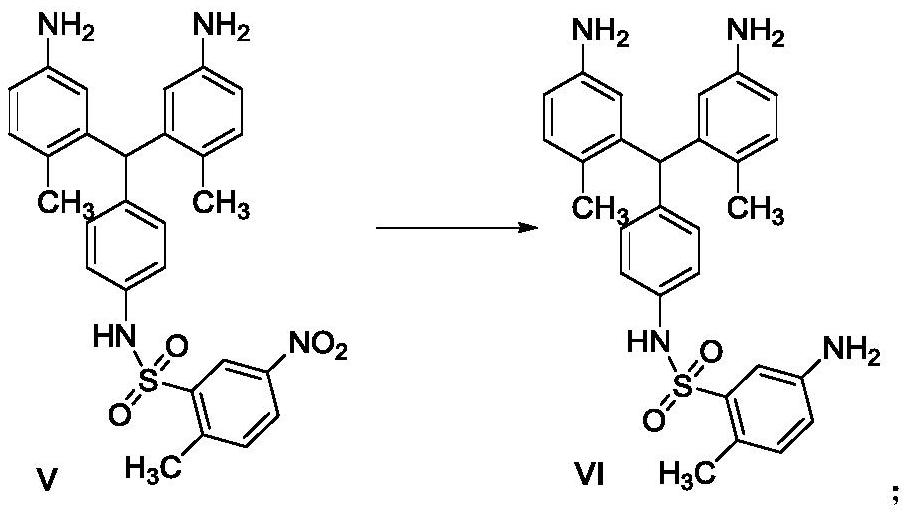
A kind of preparation method of pazopanib related substance
A compound and organic solvent technology, applied in the field of preparation of pazopanib-related substances, can solve problems such as difficult synthesis of impurities, synthesis methods of unreported impurities, etc., and achieve high purity and high synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Compound III(X 1 for the preparation of bromine):
[0063]
[0064] Under the protection of nitrogen, cool 23.8ml of isopropylmagnesium chloride-lithium chloride (1.3M / tetrahydrofuran solution) to -20°C ~ 0°C, add 8.4g of compound I, keep the reaction for 2 hours, dissolve 5.6g of compound II in 10ml of tetrahydrofuran Add it dropwise to the above system, stir and react at -20°C to 0°C for 3 hours, warm up to room temperature, add 100ml of saturated ammonium chloride aqueous solution, 100ml of ethyl acetate, stir and stand for liquid separation, and wash the organic phase with saturated brine Evaporate under reduced pressure to obtain a light yellow solid, add 100ml of petroleum ether, beat at room temperature for 2 hours, then filter, and dry the filter cake under reduced pressure to obtain 9.7g of off-white solid, namely compound III. Yield 92.6%, 1 H-NMR (400M, DMSO-d6) δ: 2.16(s, 6H), 5.89~5.90(d, J=4.0Hz, 1H), 5.93~5.95(d, J=8.0Hz, 1H), 7.15~7.17 (d, J=8.0Hz,...
Embodiment 2
[0080] Compound III(X 1 for the preparation of bromine):
[0081] Under the protection of nitrogen, cool 11.6ml of butyl lithium (1.6M / n-hexane solution) to -80°C ~ -60°C, dissolve 5.0g of compound I in 10ml of tetrahydrofuran, drop it into the above system, and keep it warm for 2 hours. 3.4 g of compound II was dissolved in 10 ml of tetrahydrofuran, added dropwise to the above system, kept stirring for 3 hours, heated to -20°C to 0°C for 2 hours, then warmed up to room temperature, added 100 ml of saturated ammonium chloride aqueous solution, ethyl acetate 100ml, stirred and left to separate liquids, the organic phase was washed with saturated brine, and then evaporated to dryness under reduced pressure to obtain a light yellow solid. Compound III. The yield is 94.8%.
[0082] Compound IV(X 1 for the preparation of bromine, hydrochloride):
[0083] 5.0 g of compound III and 2.5 g of aniline were mixed, and the temperature was raised to 100°C. Dissolve 1.8g of zinc chlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com