A kind of synthetic method of pazopanib hydrochloride crude drug trimer impurity
A technology for the synthesis of pazopanib hydrochloride and its synthesis method, which is applied in the field of synthesis of trimer impurities of pazopanib hydrochloride raw material drug, which can solve the problems of low drug impurity content, small amount of impurities, and difficulty in large-scale production, and achieve an improvement Quality standard, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
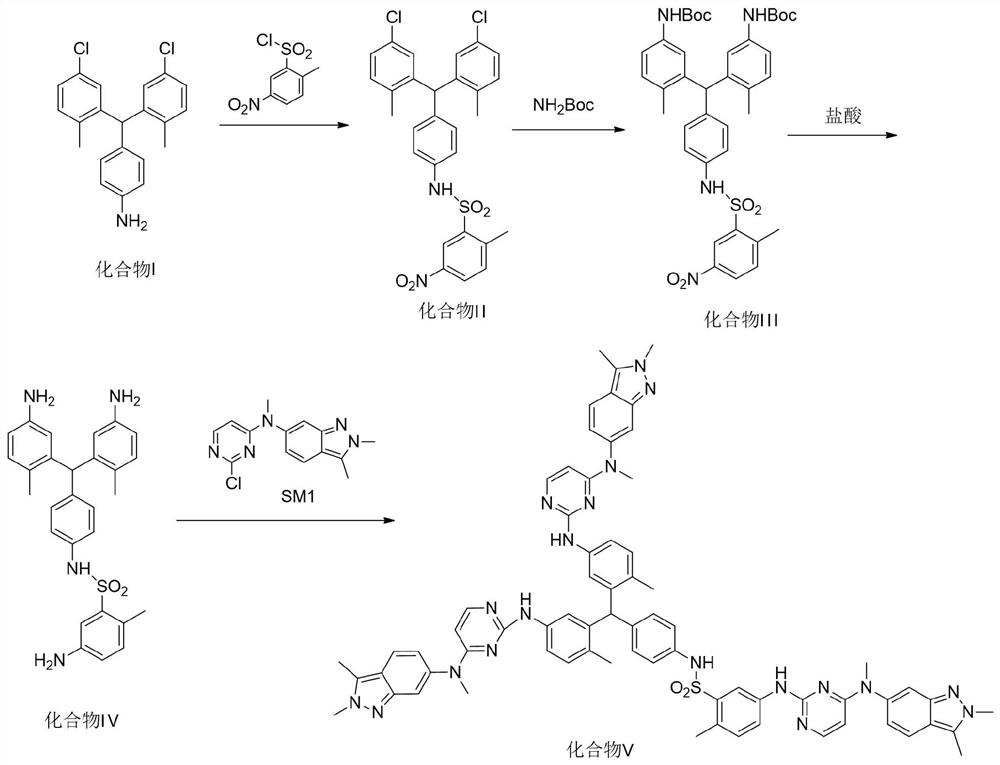
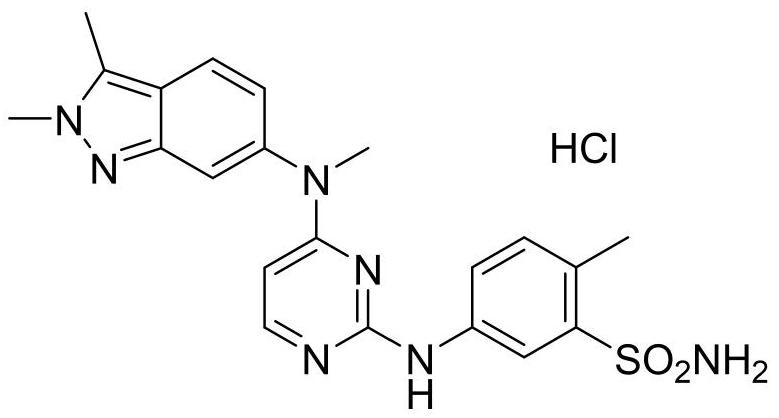
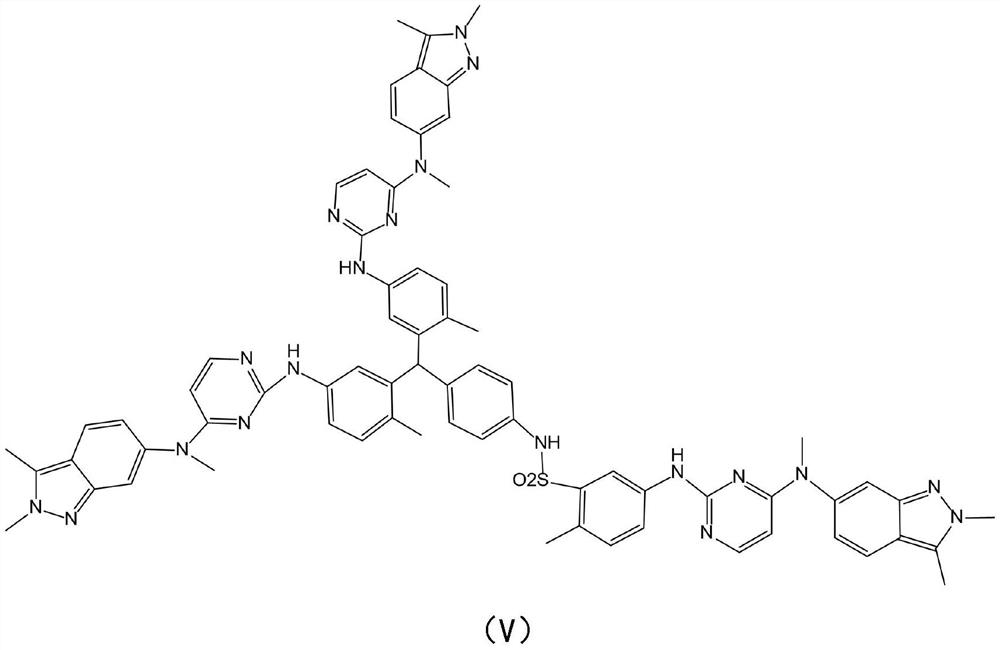
[0023] The present invention provides a kind of synthetic method of pazopanib hydrochloride crude drug trimer impurity, the structural formula of described pazopanib hydrochloride crude drug trimer impurity is following formula (V):
[0024]
[0025] figure 1 A synthetic flow diagram according to an embodiment of the invention is shown. Such as figure 1 Shown, the synthetic method of described pazopanib hydrochloride crude drug trimer impurity formula (V) comprises:
[0026] Step 1: take compound I, make it condense with 2-methyl-5-nitrobenzenesulfonyl chloride to obtain compound II;
[0027] Specifically, the solvent for the condensation reaction includes one or more of acetone, tetrahydrofuran, and dioxane. Preferably, the solvent for the condensation reaction is tetrahydrofuran, and the acid-binding agent for the condensation reaction includes triethylamine, One or more of pyridine, sodium hydroxide, potassium hydroxide and potassium carbonate, preferably, the acid-bi...
Embodiment example 1
[0036] Embodiment case one, the synthetic method of compound II is:
[0037] Take a 100mL two-necked reaction flask, add 4.05g of compound I, 40ml of tetrahydrofuran and 5mL of pyridine respectively, and add 2-methyl-5-nitrobenzenesulfonyl chloride (2.95g, 12.51mmol, 1.1eq), and then at room temperature Then, add ethyl acetate and water to the reaction solution, extract and separate the liquid, wash the organic phase obtained after the liquid separation with saturated brine, dry over anhydrous sodium sulfate, filter, and rotary evaporate to dryness ; The obtained crude product was purified by silica gel column to obtain 3.85g yellow solid; yield: 61%.
[0038] The above reaction formula is:
[0039]
Embodiment example 2
[0040] Embodiment two, the synthetic method of compound III is:
[0041] Take a 100mL two-necked reaction flask, under nitrogen protection, add 3.85g of compound II, 50ml of tetrahydrofuran, 0.1g of triphenylphosphine palladium, 2.50g of potassium carbonate and an appropriate amount of tert-butyl carbamate, and heat up to 70°C After the reaction, the solvent was removed by rotary evaporation, and then ethyl acetate and water were added for extraction and liquid separation. After the liquid separation, the organic phase obtained was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and rotary evaporated to dryness. The obtained crude product was purified by silica gel column to obtain 5.07 g of yellow solid; yield: 98%.
[0042] The above reaction formula is:
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com