Method for detecting pazopanib drug concentration in human plasma by LC-MS/MS (liquid chromatography-mass spectrometry/mass spectrometry)
A technology of pazopanib and drug concentration, applied in the field of drug concentration analysis, to achieve the effects of small introduction error, good separation effect, and improved precision and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
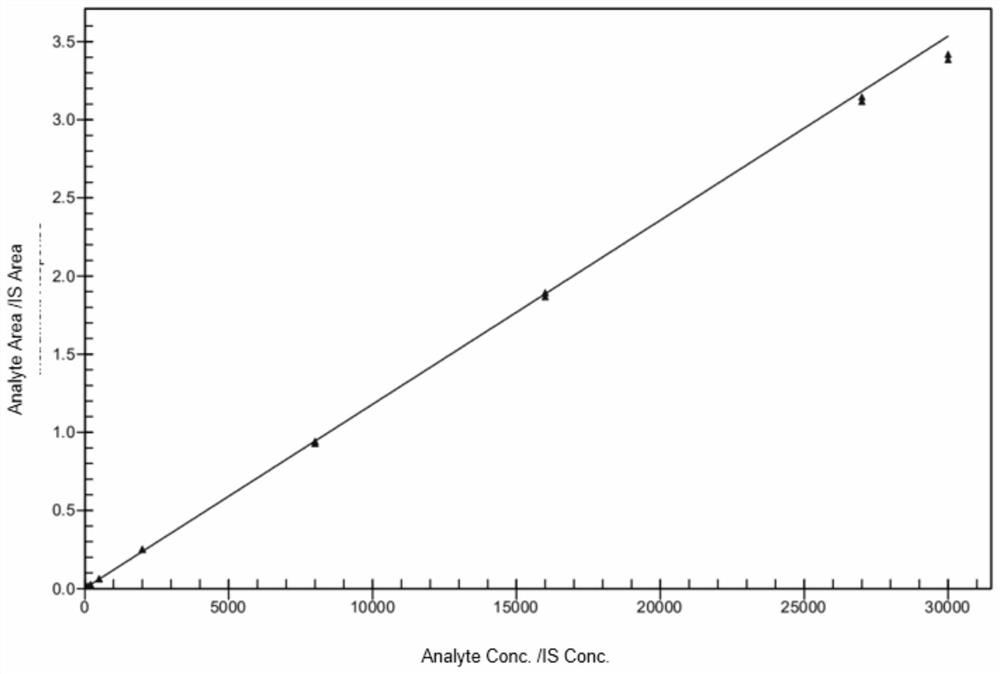
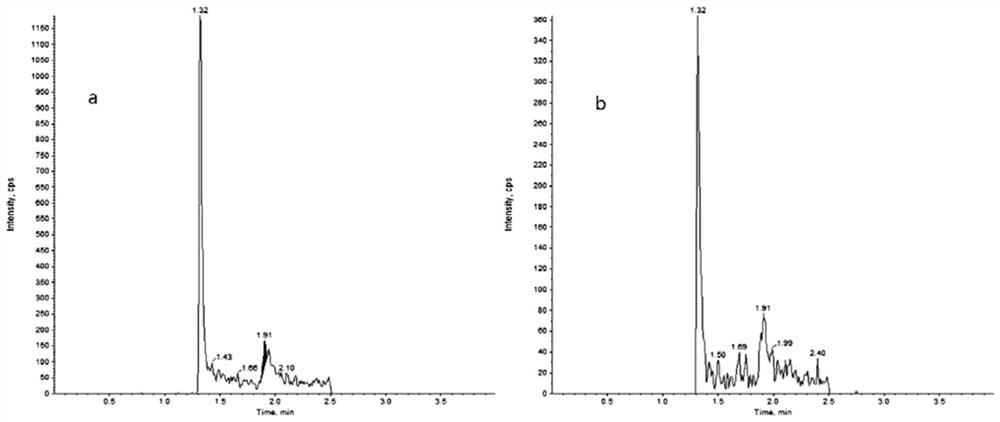
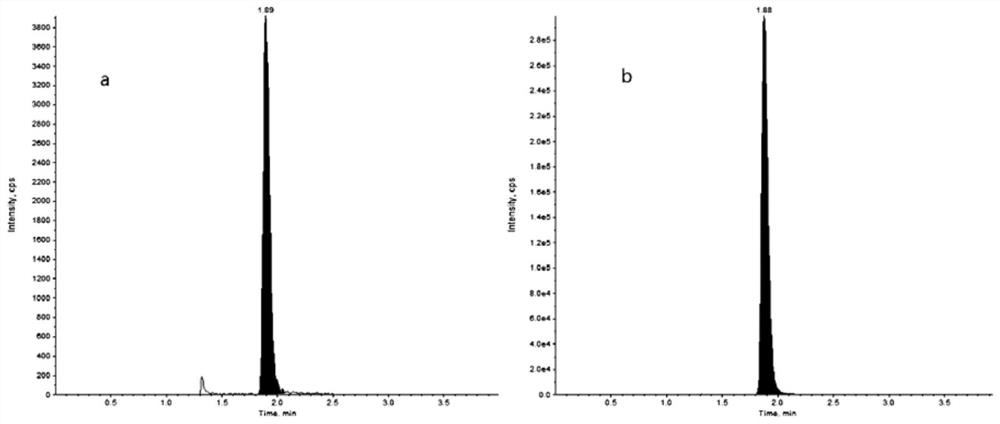
[0037]In order to solve the technical problem that the prior art does not involve the detection of the drug concentration of pazopanib in human plasma, the present invention provides a LC-MS / MS combined method for detecting the drug concentration of pazopanib in human plasma. The detection method includes taking a series of pazopanib working solutions and mixing them with human blank plasma respectively, injecting them into LC-MS / MS for analysis after pretreatment, and adding a compound mainly composed of pazopanib-d6 to the human plasma to be tested. After pretreatment, the internal standard working solution was also injected into LC-MS / MS for analysis, with the chromatographic peak area ratio of pazopanib and internal standard pazopanib-d6 as the ordinate, and the ratio of pazopanib in human blank plasma The concentration of pazopanib is the abscissa to make a standard curve, and the concentration of pazopanib in the human plasma sample to be tested is calculated according to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com