Diagnostic and therapeutic methods for sarcomatoid kidney cancer
A therapy, sarcoma technology, applied in the field of diagnosis and treatment of sarcomatoid renal cell carcinoma, which can solve problems such as difficulty in detection and treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0656] Example 1: Sarcomatoid Histology, MSKCC Risk Score, and Molecular Correlations Differentiate Response to Atezolizumab + Bevacizumab vs. Sunitinib: Stage III of Untreated Metastatic Renal Cell Carcinoma Research (IMmotion151) Results
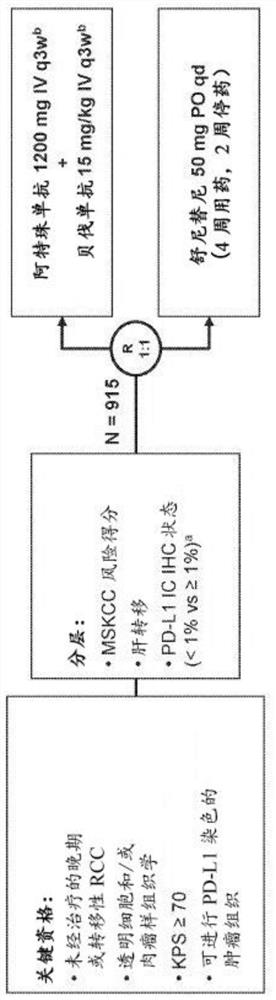
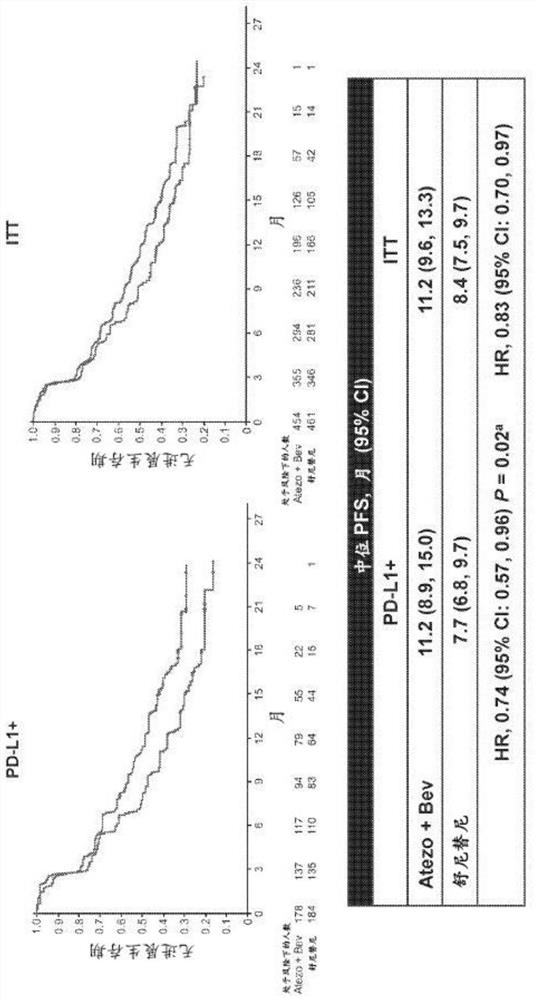
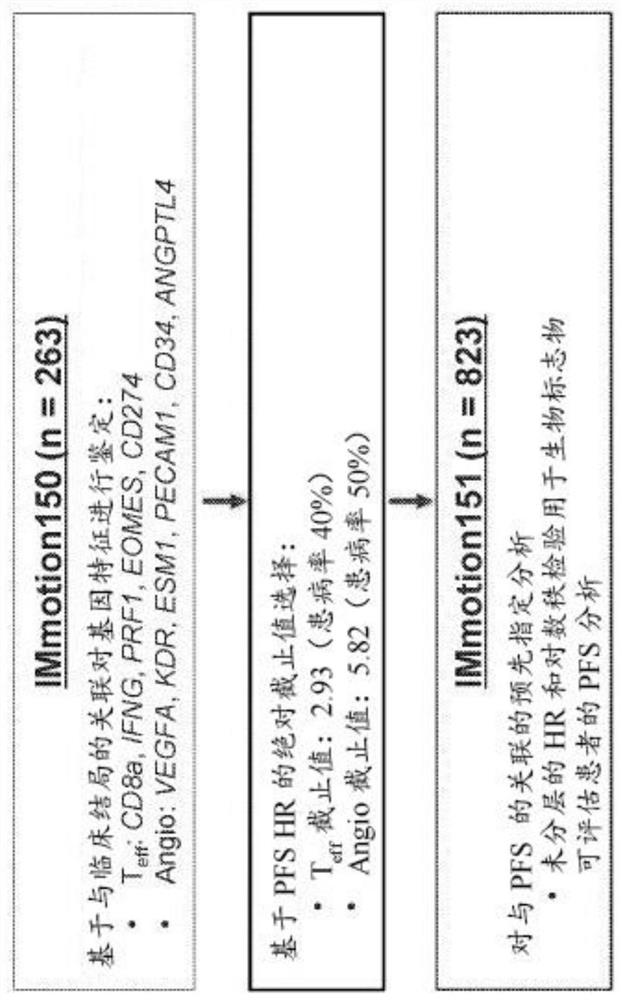
[0657] The IMmotion151 study (ClinicalTrials.gov identifier NCT02420821) is a multicenter, randomized, open-label study evaluating atezolizumab in combination with bevacizumab and sunitinib in patients with inoperable, locally advanced, or metastatic disease Efficacy and safety in patients with sexual RCC who have not received prior systemic active or experimental therapy in the adjuvant or metastatic setting. see figure 1 . The co-primary endpoints of the study were PFS in the PD-L1+ subgroup and OS in the ITT population. Exploratory endpoints included biomarker characterization in sarcoid-like tumors and MSKCC risk subgroups, as well as validation of gene signatures from the IMmotion150 study and association of gene signatures with PF...
example 2
[0669] Example 2: Atezolizumab + Bevacizumab vs Sunitinib in Patients with Untreated Metastatic Renal Cell Carcinoma and Sarcomatoid Histology: IMmotion151 Subgroup Analysis
[0670] As described in Example 1, renal cell carcinoma (RCC) with sarcomatoid histology is characterized by the presence of spindle-shaped malignant lesion epithelial cells. Sarcomatoid histology is associated with multiple histological subtypes of RCC and confers an aggressive phenotype. Patients with metastatic RCC with sarcomatoid histology (approximately 10%-20% of patients with advanced disease) have a particularly poor prognosis and have a limited response to vascular endothelial growth factor pathway inhibition. Here, we report the results of a prespecified subgroup analysis to assess the efficacy of atezolizumab plus bevacizumab versus sunitinib in IMmotion151-enrolled patients with sarcoid histology and explore Biological correlates of sarcomatoid and non-sarcomatoid histology.
[0671] method...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com