Preparation method of pazopanib hydrochloride
A technology of pazopanib mono-hydrochloride, which is applied in the field of drug synthesis, can solve the problems of not being able to meet the needs of medicine, and achieve the effects of improving the sticking phenomenon, less residual solvent, and low bulk density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
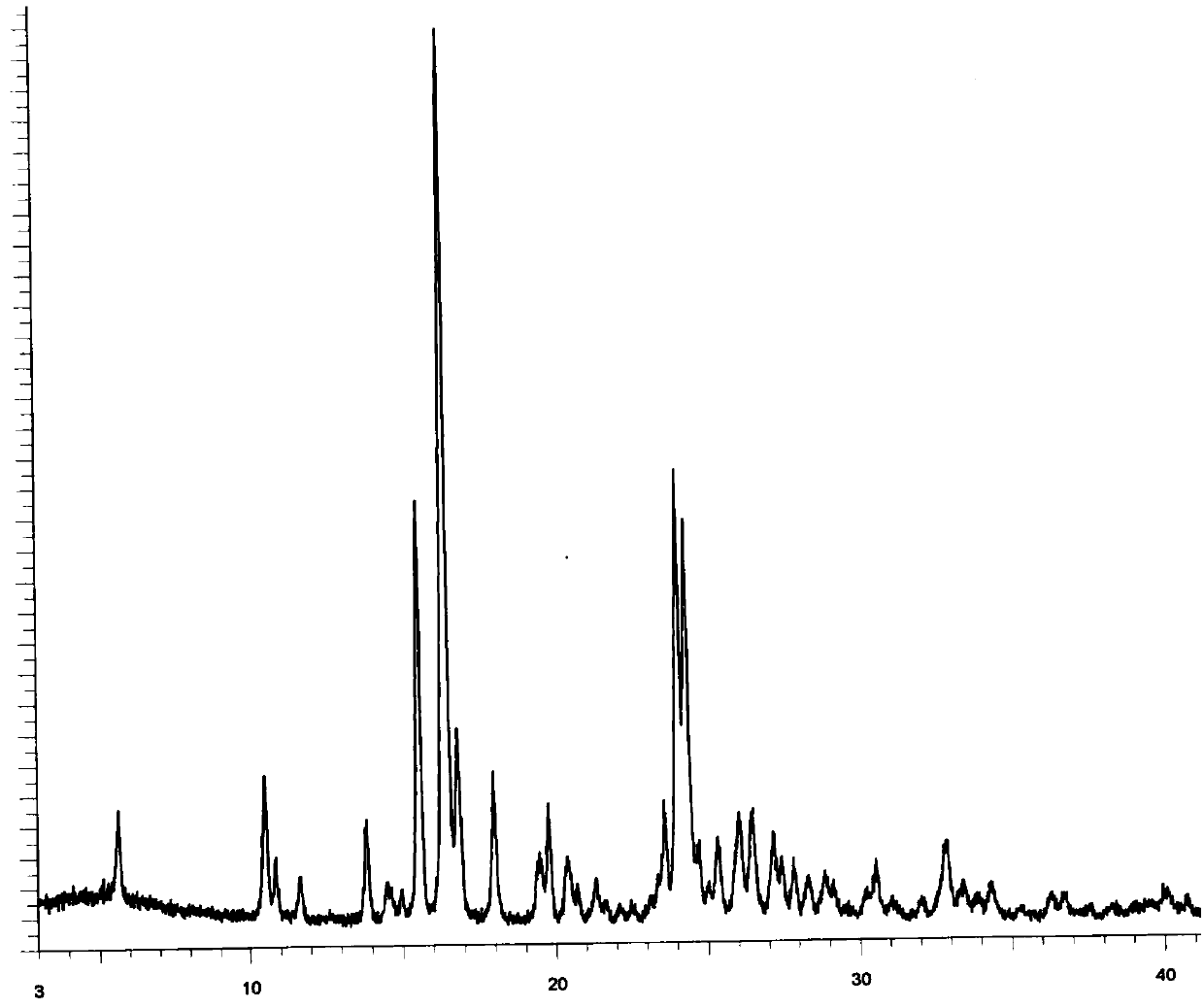
[0016] Put 100g of pazopanib monohydrochloride hydrate in 700ml of acetonitrile-water mixed solution (volume ratio 30:1), stir at 60-70°C for 8-10 hours, cool to room temperature and stir for 1-1.5 hours and filter, 70- After vacuum drying at 80°C for 17-18 hours, 95.2 g of pazopanib monohydrochloride was obtained, yield: 95.2%. GC detection showed that the residue of acetonitrile was 76 ppm, and its XRD was as follows: figure 1 , the microscope photo as figure 2 , The bulk density is 0.22g / ml.
Embodiment 2
[0018] Fully according to the operation method of implementation one, adjust the volume ratio of the acetonitrile-water mixed solution and repeat the experiment. The specific parameter adjustment and results are as follows:
[0019]
Embodiment 3
[0021] Put 100g of pazopanib monohydrochloride hydrate in 2000ml of acetonitrile-water mixed solution (volume ratio 30:1), stir at 50-60°C for 6 hours, cool to room temperature and stir for 1-1.5 hours, filter, 70-80°C After vacuum drying for 17 to 18 hours, 93.5 g of pazopanib monohydrochloride was obtained, yield: 93.5%, and GC detection showed that the residue of acetonitrile was 82 ppm, and its XRD was as follows: figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com