Novel crystal form of ibrutinib and preparation method of novel crystal form
A technology of ibrutinib and crystal form, applied in the field of chemical pharmacy, can solve the problems of solubility, stability difference, influence on drug bioavailability, curative effect and safety, etc., achieve low cost, good clinical application value, guarantee The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Example 1: Preparation of Ibrutinib powder used as raw material for the preparation of the new crystal form (crystal form 1) of the present invention
[0121] Prepared by the same method as CN101610676A Example 1b: add 3.86g of intermediate (R)-3-(4-phenoxyphenyl)-1-(piperidin-3-yl)-1H to a 200mL three-necked flask -pyrazolo[3,4-d]pyrimidin-4-amine and 35ml of dichloromethane, start stirring. Add 4.2 mL of triethylamine, slowly add 1 mL of acryloyl chloride dropwise, and continue the reaction for 2 hours after the addition. After the reaction, the organic phase was washed with 5% citric acid and saline solution to separate layers. MgSO for organic layer 4 After drying, the residue was purified by column chromatography (dichloromethane / methanol=25 / 1) to obtain 2.15 g of ibrutinib powder.
Embodiment 2
[0122] Example 2: Ibrutinib (1-((R)-3-(4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidine-1 -yl)piperidin-1-yl)prop-2-en-1-one) new crystal form (crystal form 1)
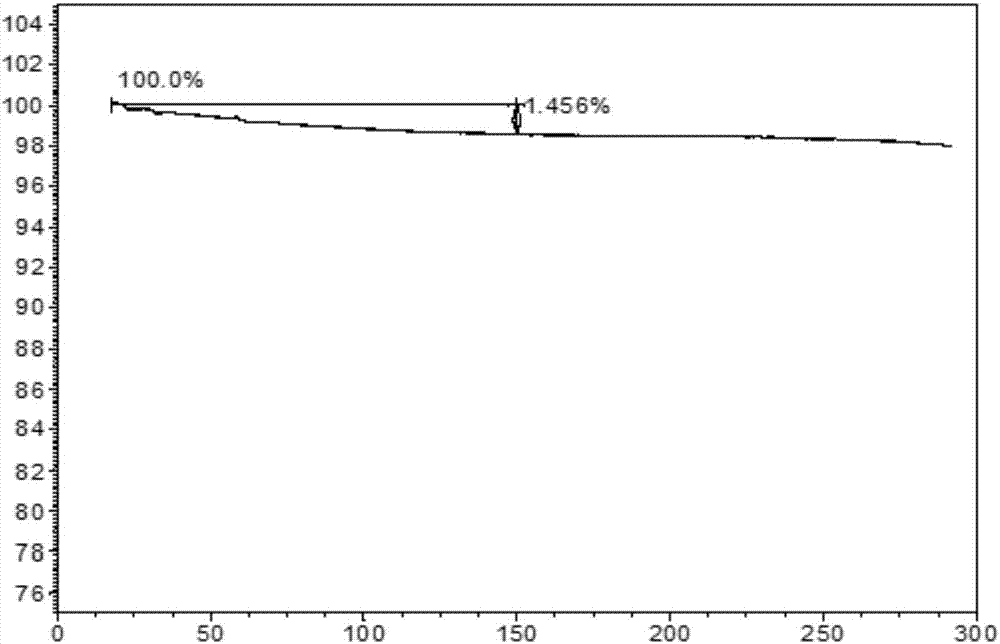
[0123] Add 500 mg of the ibrutinib powder prepared in Example 1 into 4 ml of methanol, heat to 60° C. and stir for 1 hour to obtain a clear solution. The solution was cooled to 25°C, 2 mL of water was added dropwise, and after stirring at 25°C for 1 hour, the temperature was lowered to 5°C within 1 hour and stirring was continued for 48 hours, filtered, and the solid was collected and vacuum dried at 50°C for 18 hours to obtain 466mg of crystal form 1, its XRPD pattern is as follows figure 1 shown.
Embodiment 3
[0124] Embodiment 3: Preparation of ibrutinib crystal form 1
[0125]Add 500 mg of the ibrutinib powder prepared in Example 1 into 4 ml of methanol, heat to 50° C. and stir for 2 hours to obtain a clear solution. The solution was cooled to 25°C, 2 mL of water was added dropwise, and after stirring at 25°C for 2 hours, the temperature was lowered to 5°C within 1 hour and stirring was continued for 16 hours, filtered, and the solid was collected and vacuum-dried at 50°C for 18 hours to obtain 458 mg of crystal form 1, the XRPD pattern of which is the same as that of the product prepared in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com