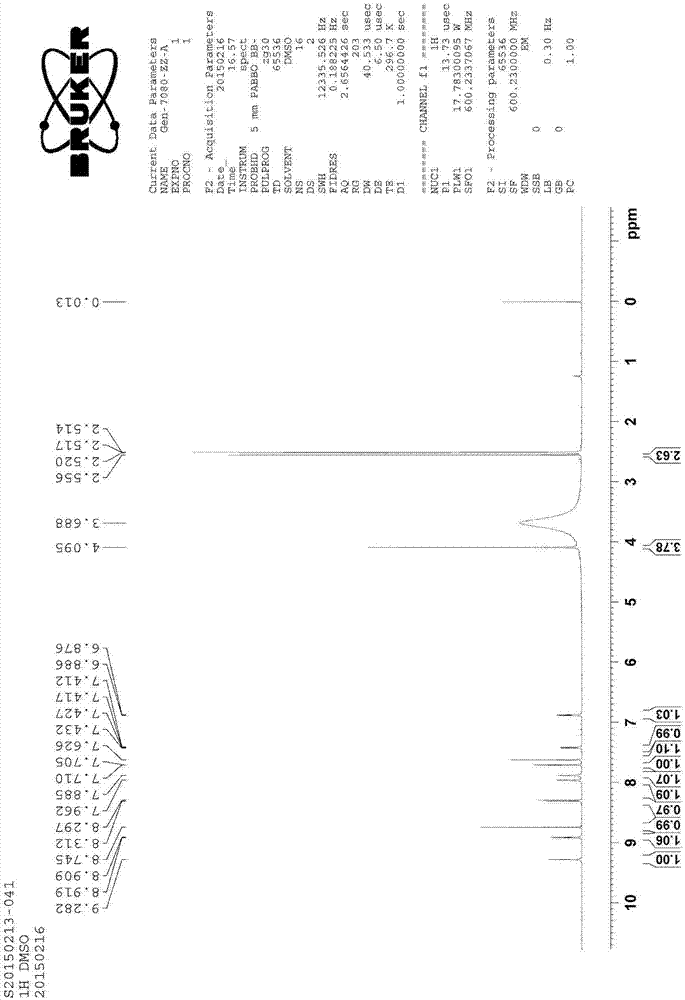
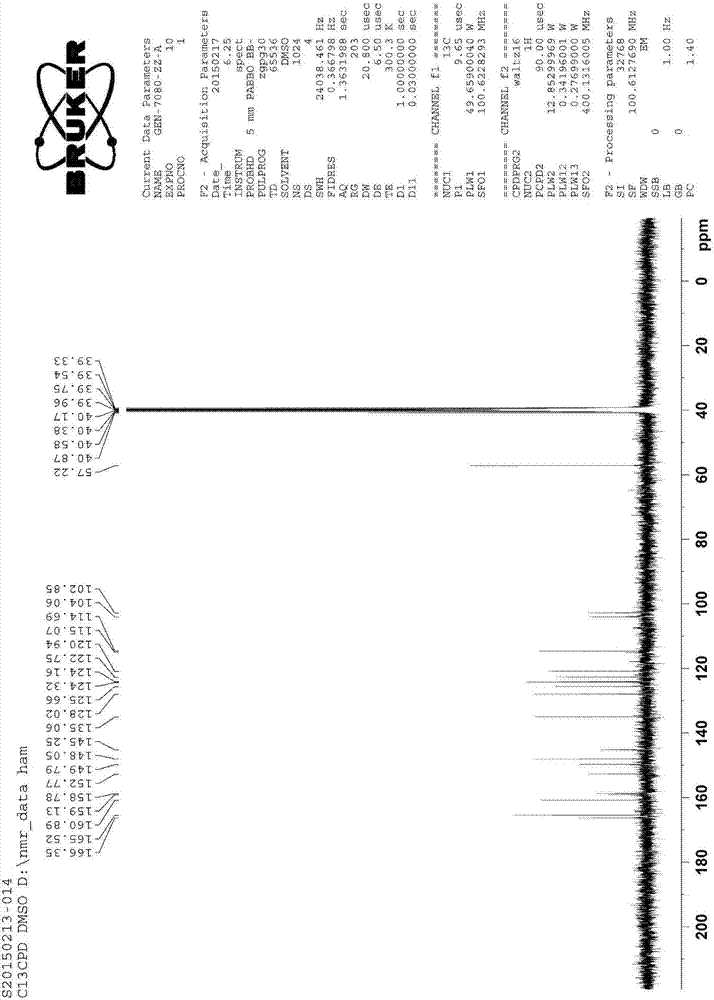
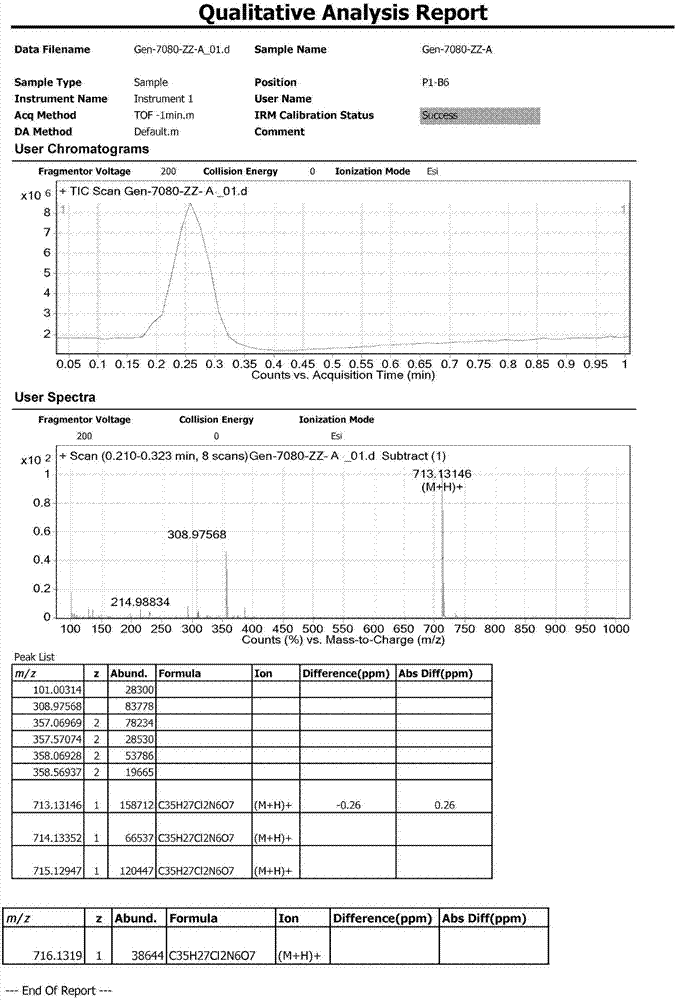
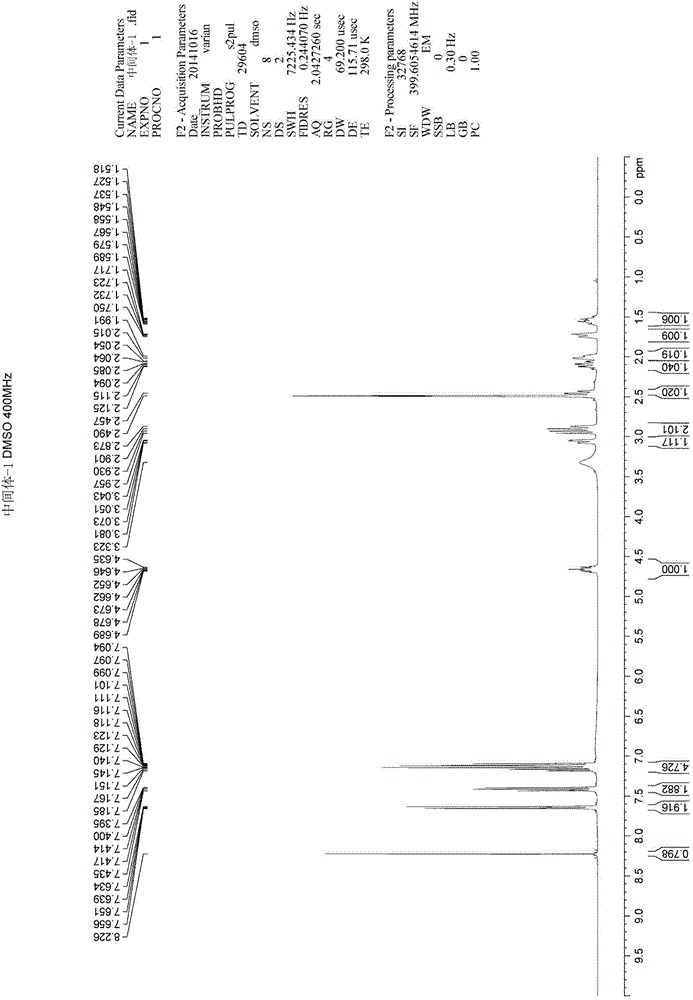
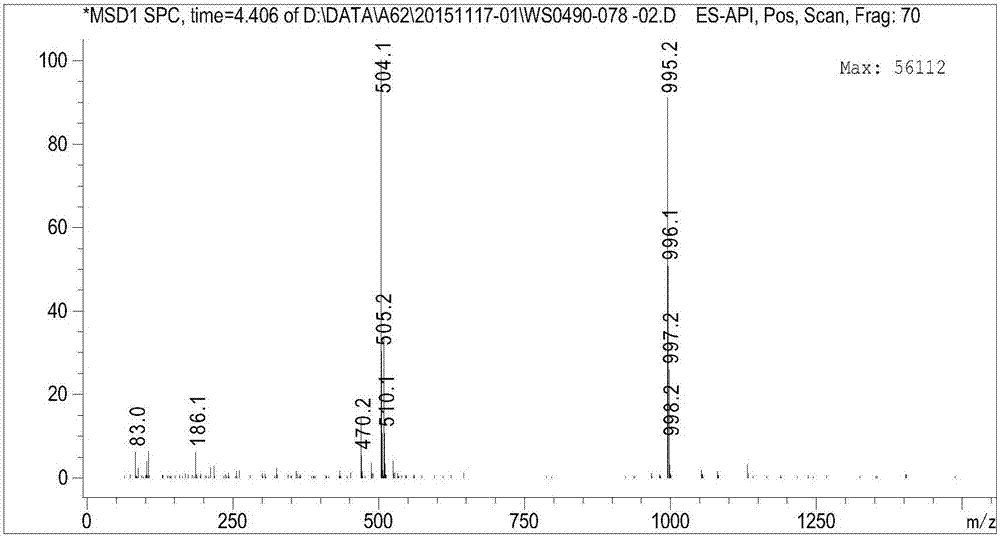
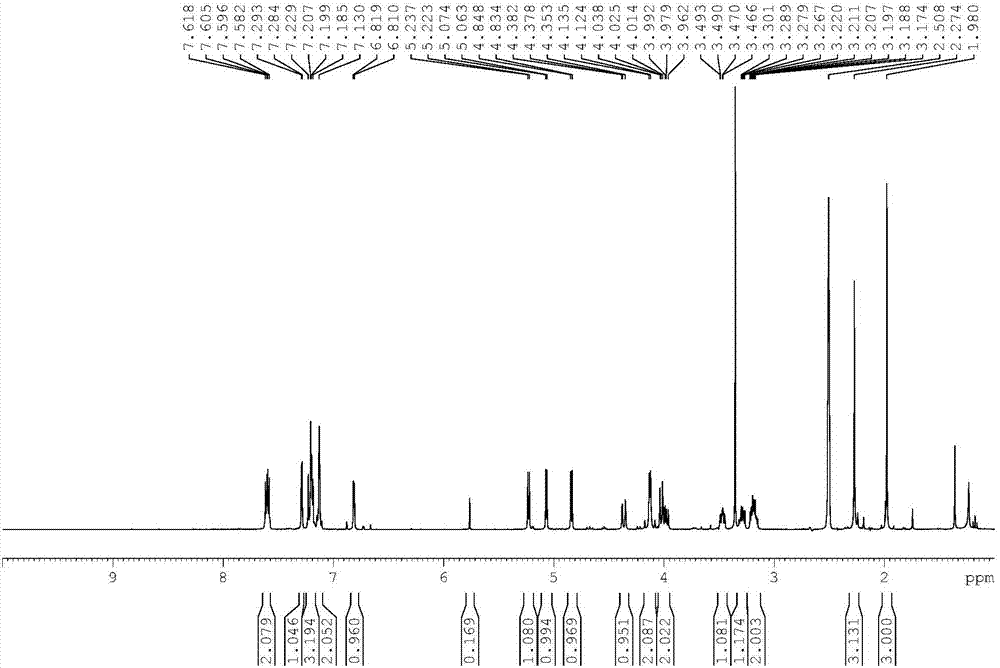
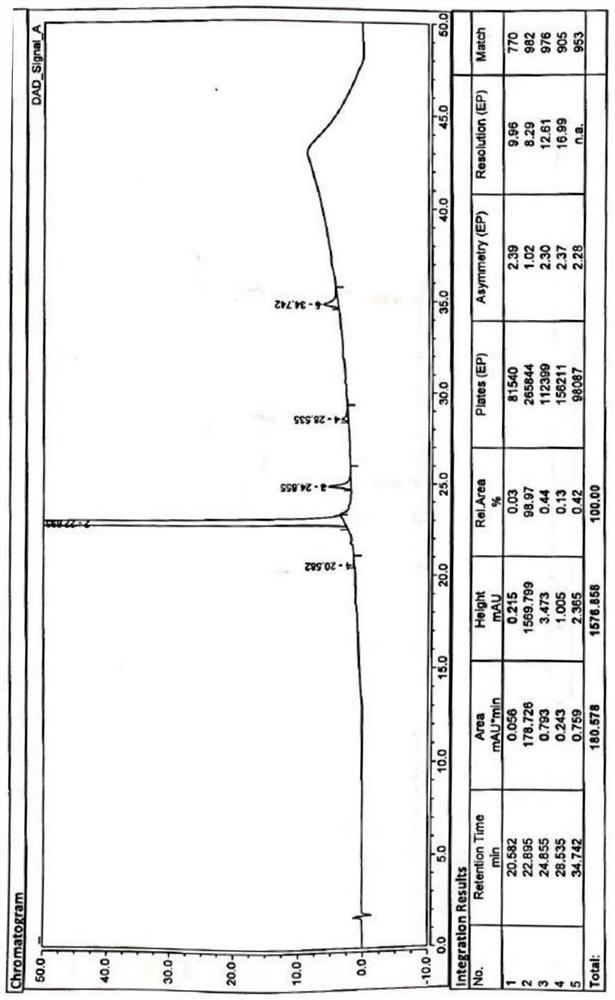
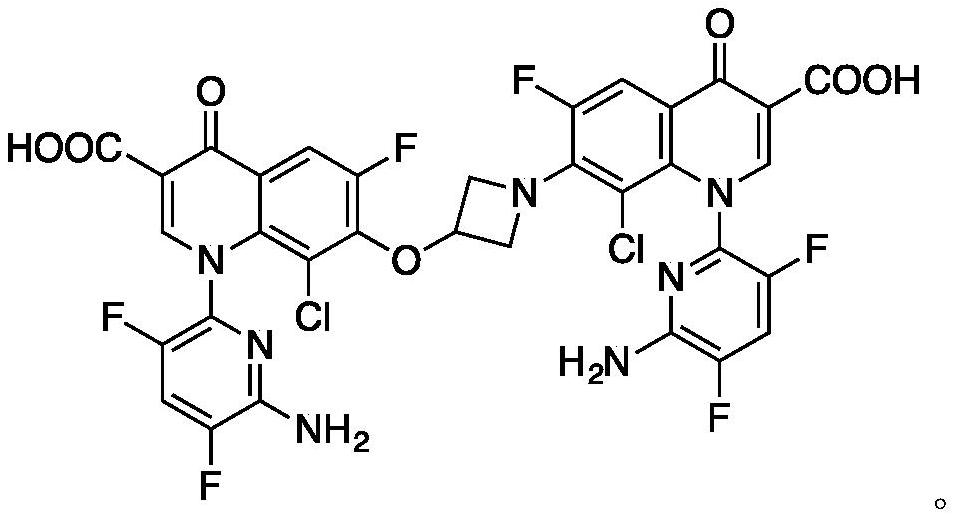
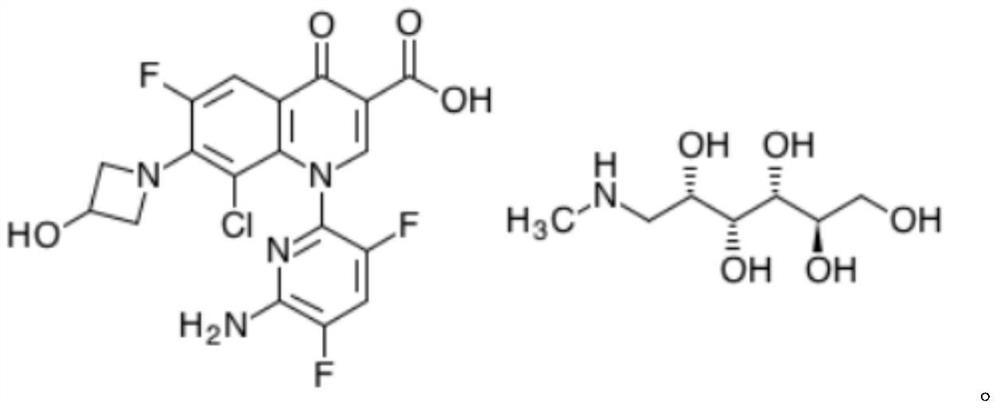
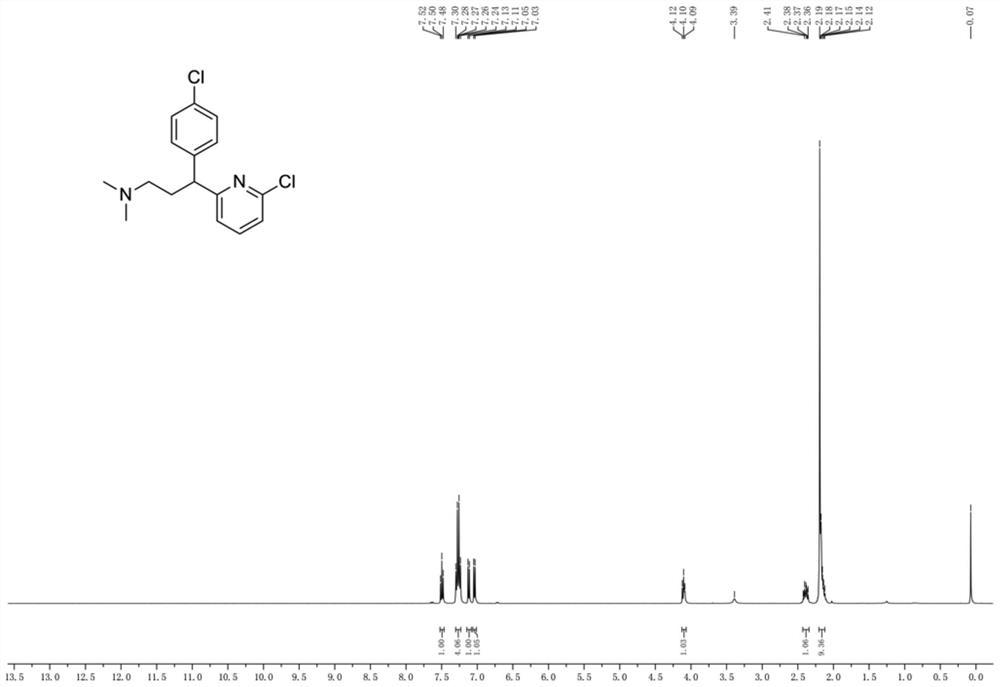
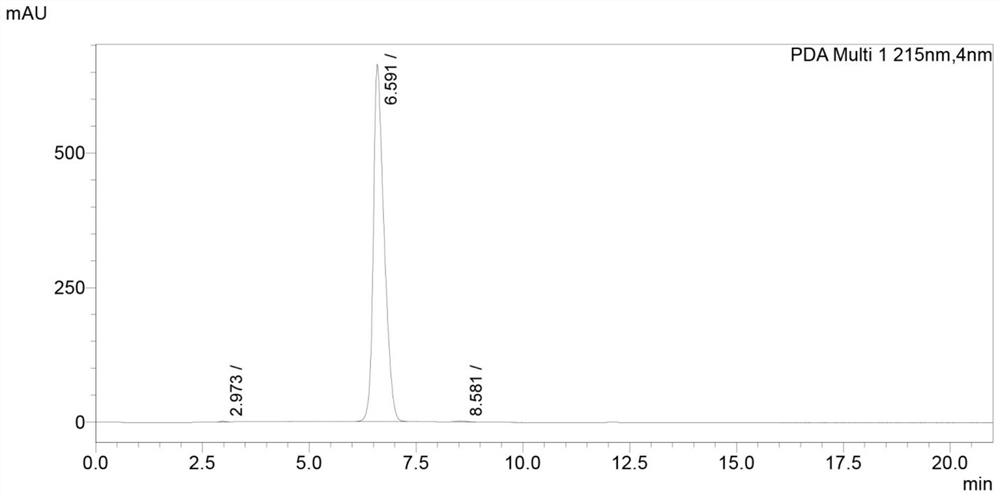
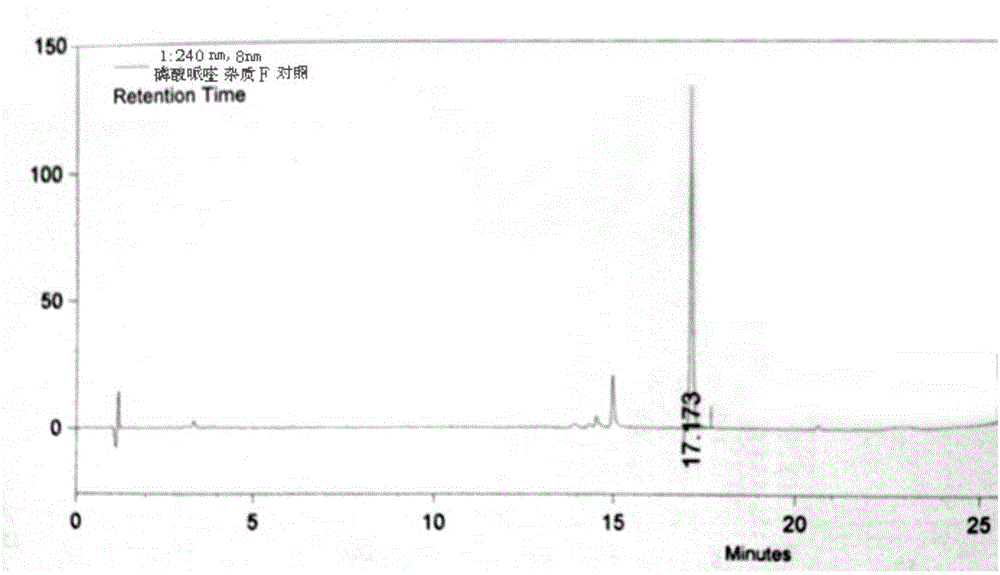
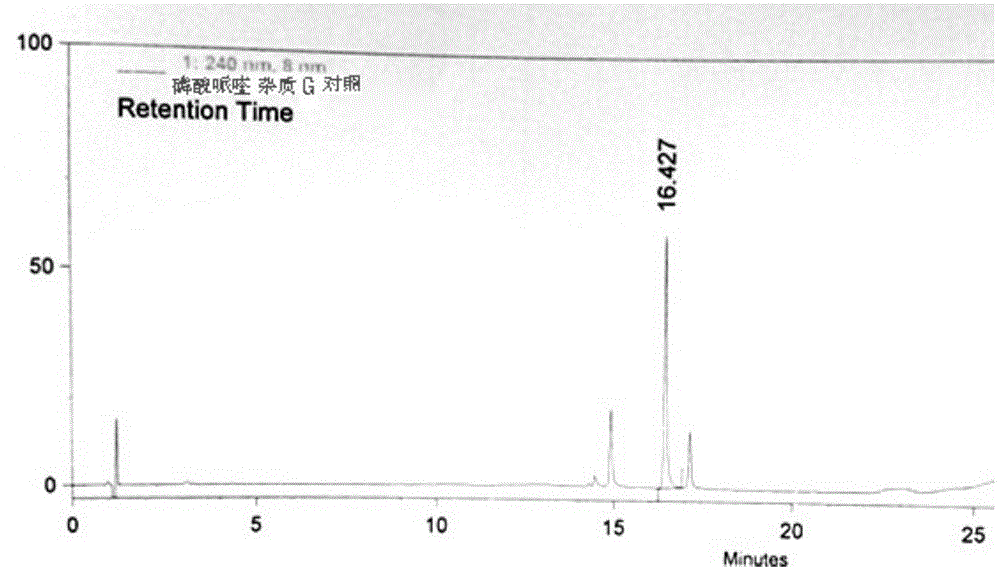
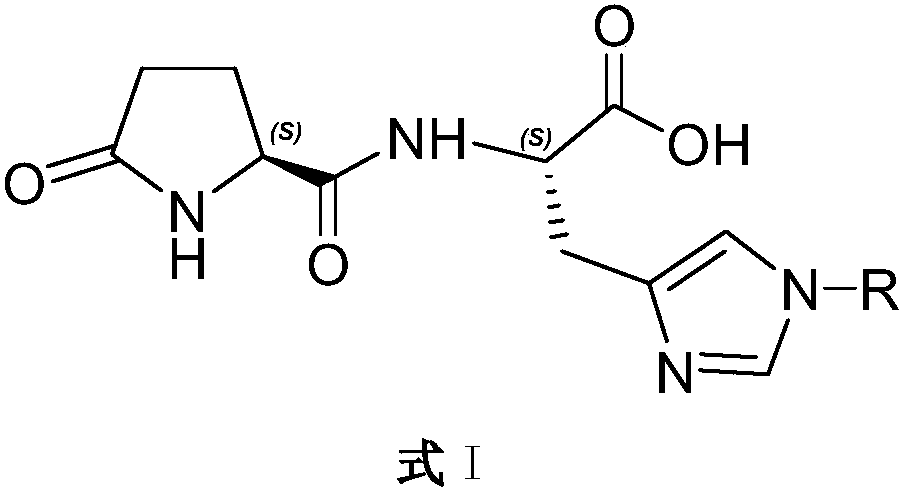
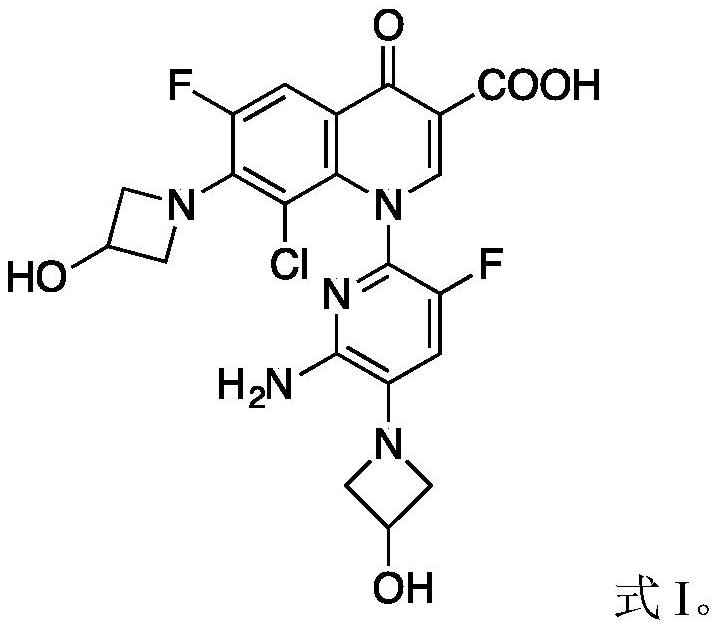
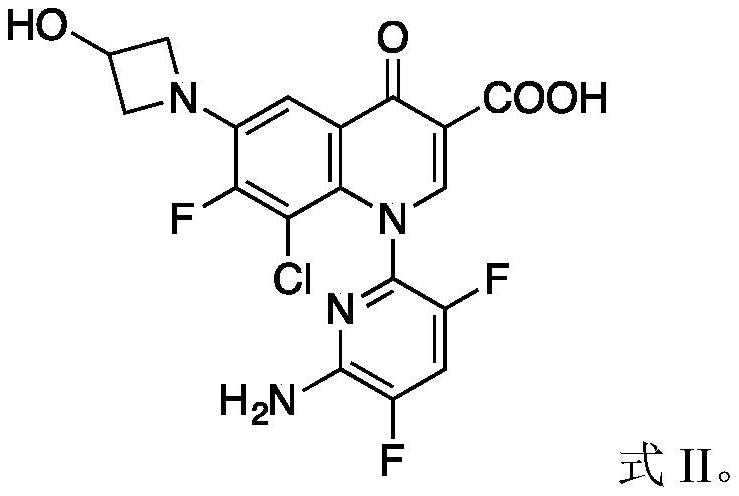
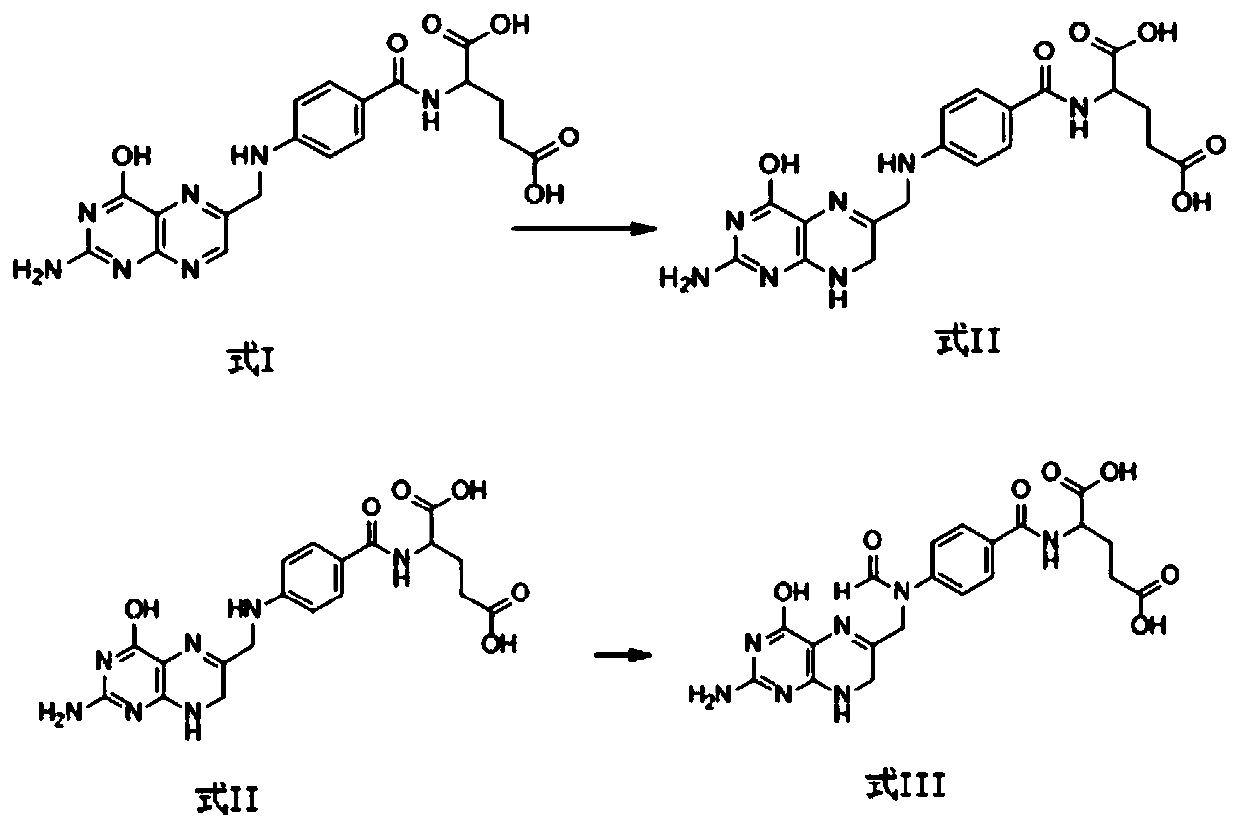
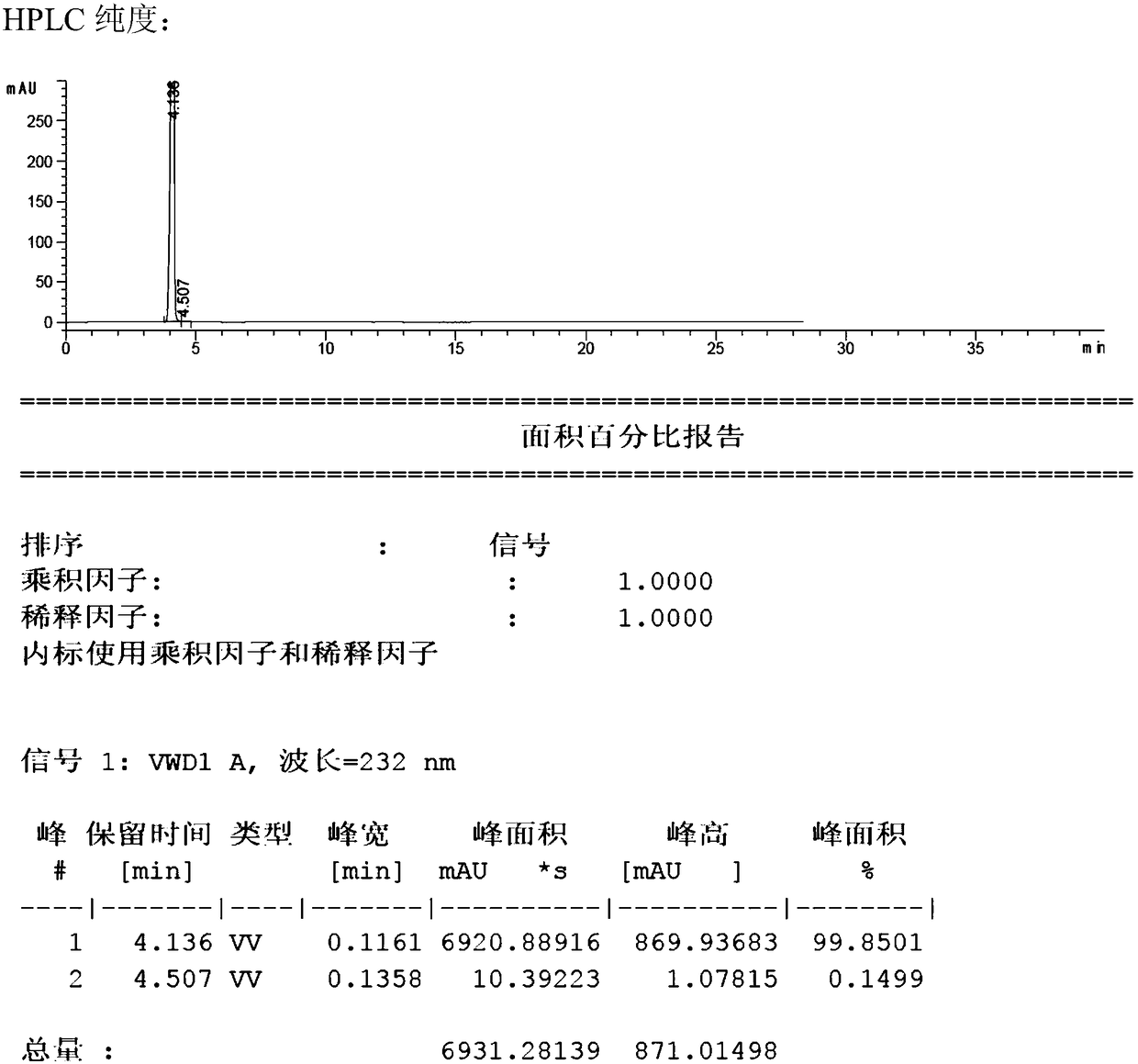
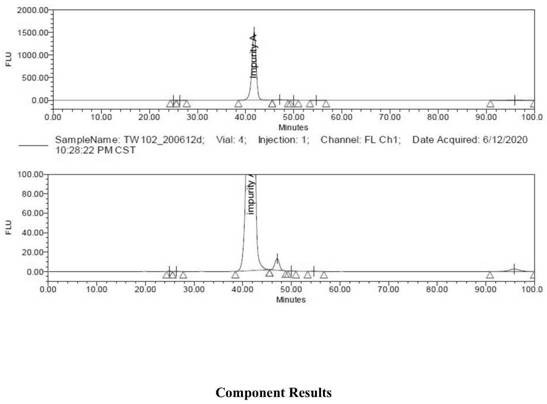
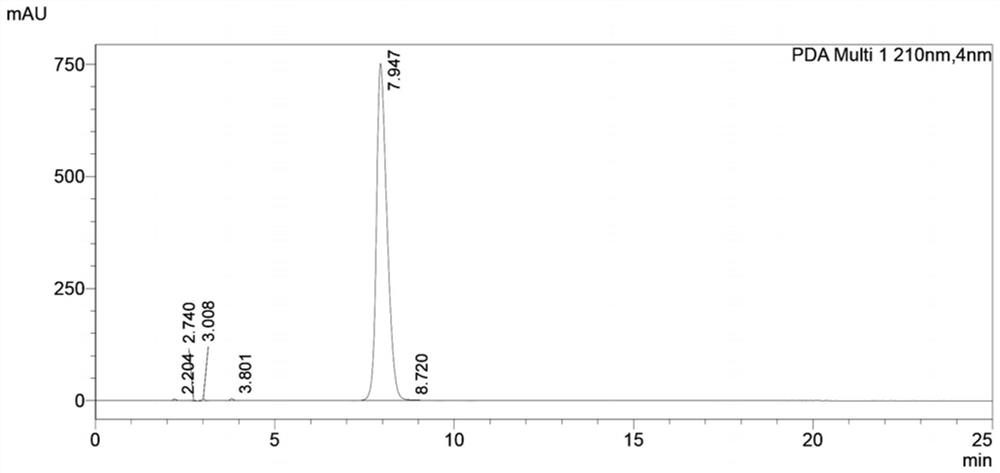
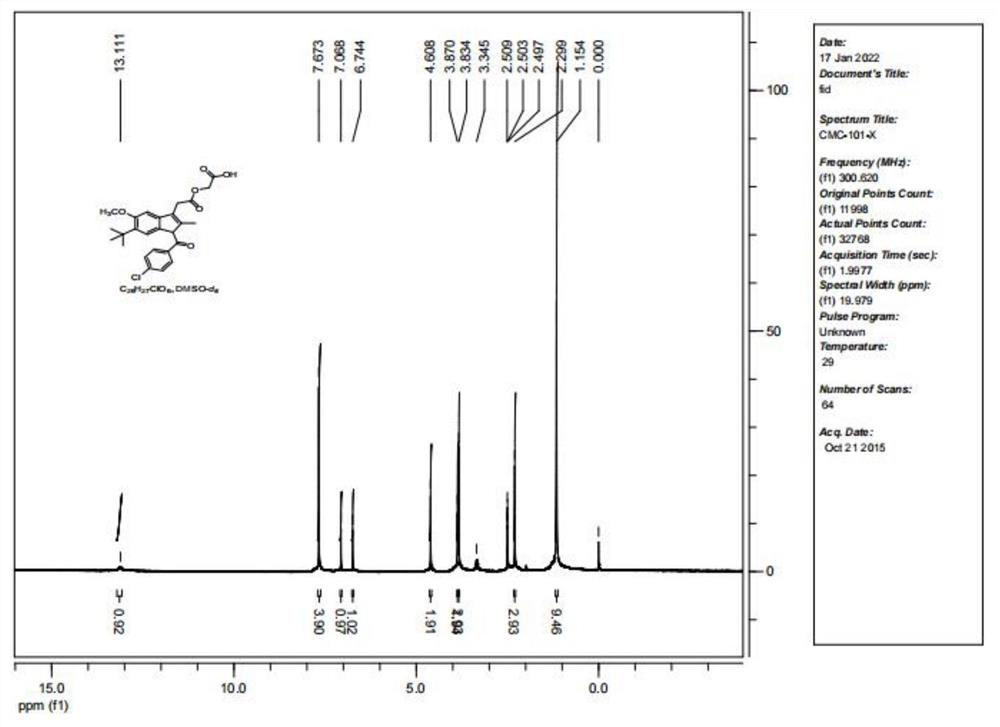
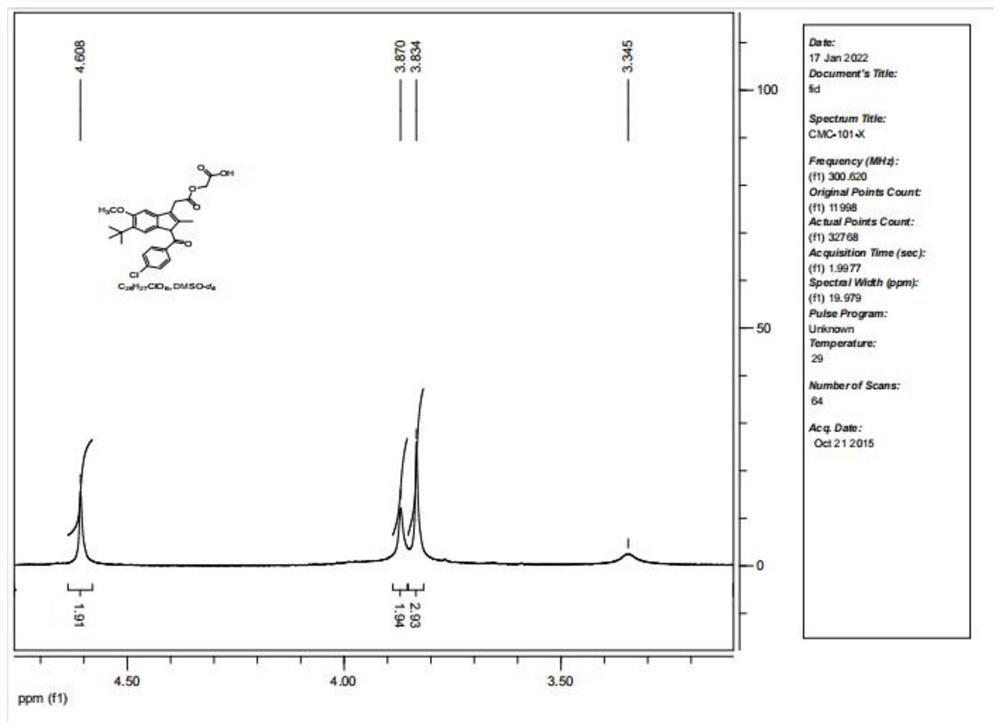
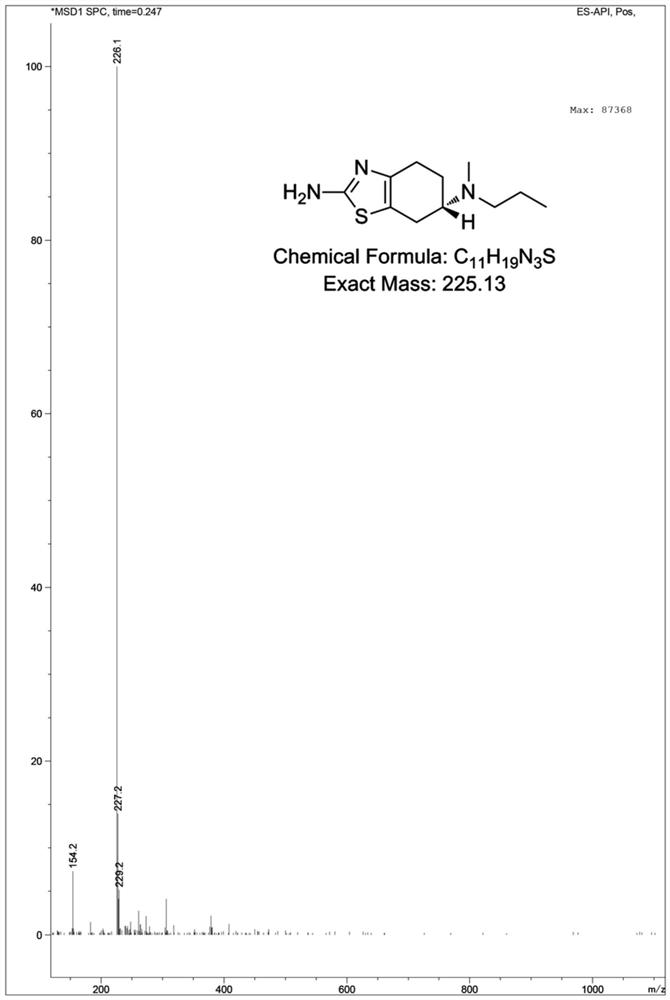
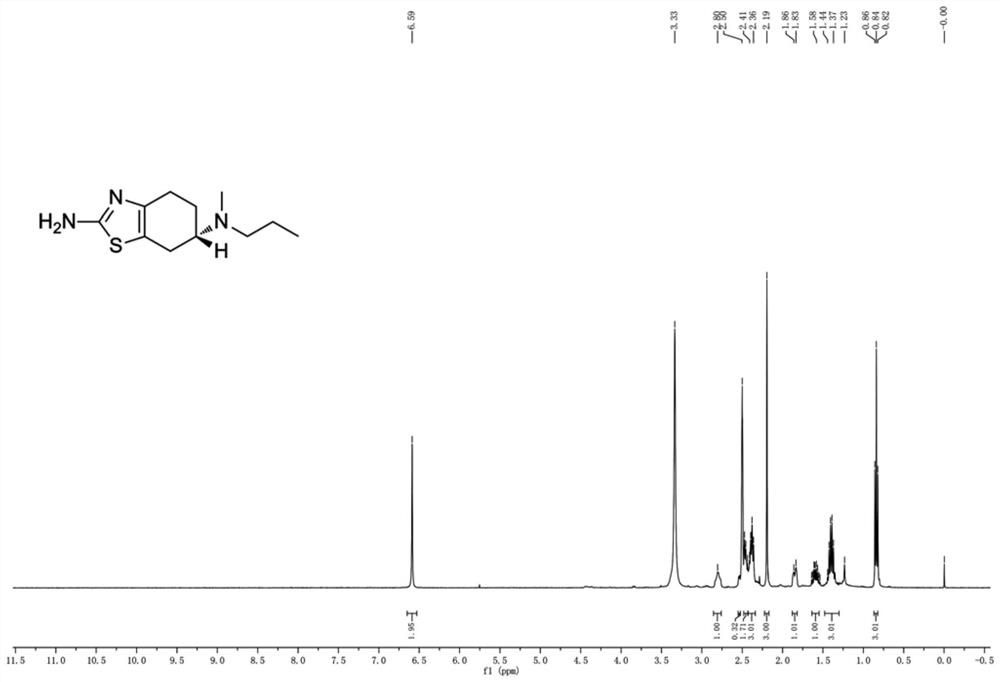
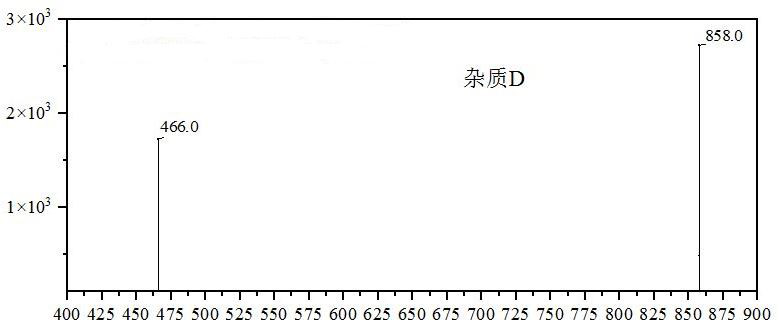
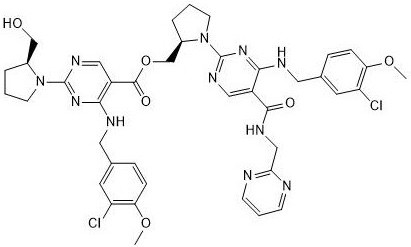
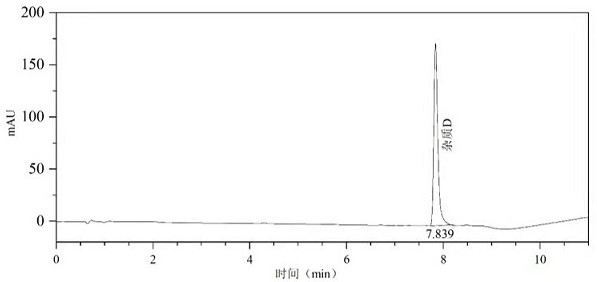
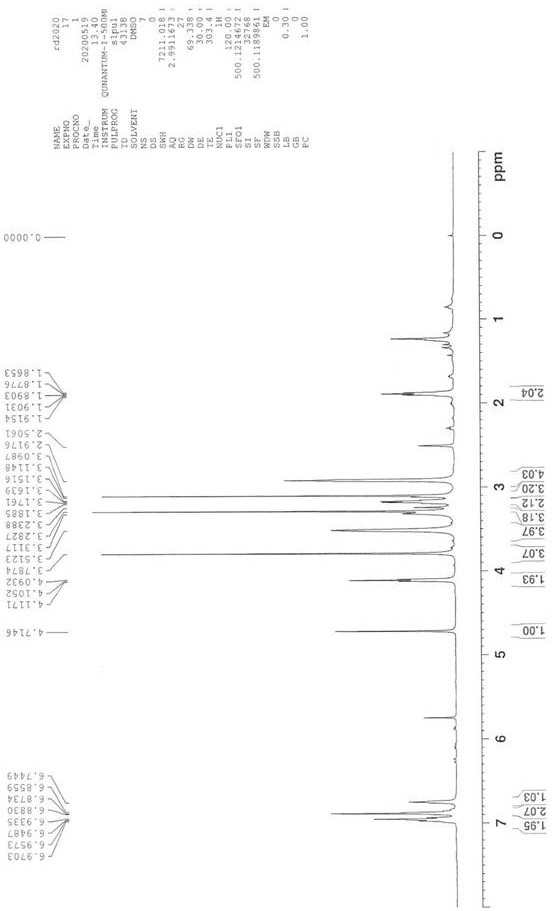
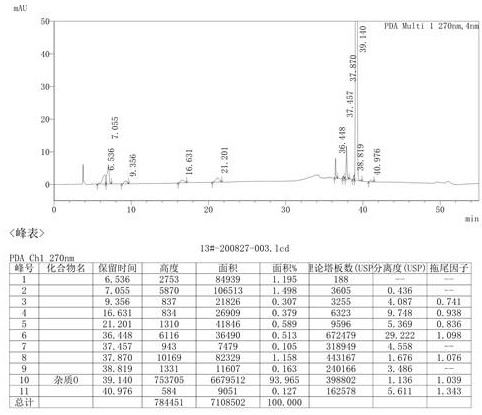
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49 results about "Drug Impurity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The presence of chemical substance other than the desired pharmaceutical compound manufactured.
Preparation methods of lenvatinib mesylate drug impurities
The invention belongs to the field of pharmaceutical synthesis, and relates to impurities in a raw medical material production process and preparation methods of the impurities, in particular to preparation methods of process impurities A, B and C of lenvatinib mesylate, namely, 4-[3-chloro-4-(N'-cyclopropylureido) phenoxy]-7-methoxyquinoline-6-carboxamide mesylate), as a drug for treating radioiodine-refractory thyroid cancer and an application of the impurities to quality research of lenvatinib mesylate. With adoption of the methods, the process impurities A, B and C are obtained through chemical synthesis for the first time, and the target compounds shown in the description can be obtained through efficient and rapid separation.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +2
Preparation method of Ibrutinib drug impurity
The invention belongs to the field of pharmaceutical synthesis, and relates to an impurity in the bulk pharmaceutical chemical production process and a preparation method of the impurity, in particular to a process impurity of Ibrutinib 1-[(3R)-3-[4-amino-3-(4-phenoxyphenyl)-1Hpyrazole [3,4-d]pyrimidine-1-yl]-1-piperidyl]-2-propylene-1-ketone and a preparation method of the process impurity.
Owner:BEIJING CREATRON INST OF PHARMA RES CO LTD
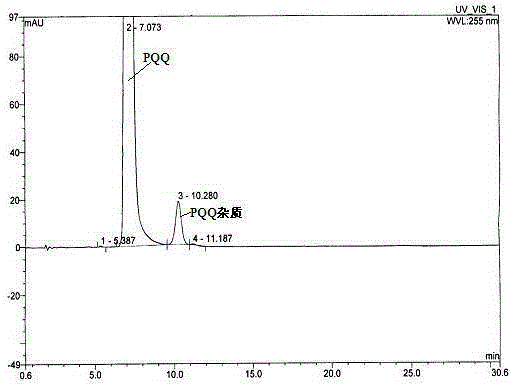
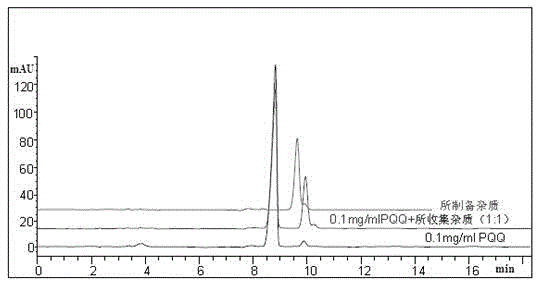
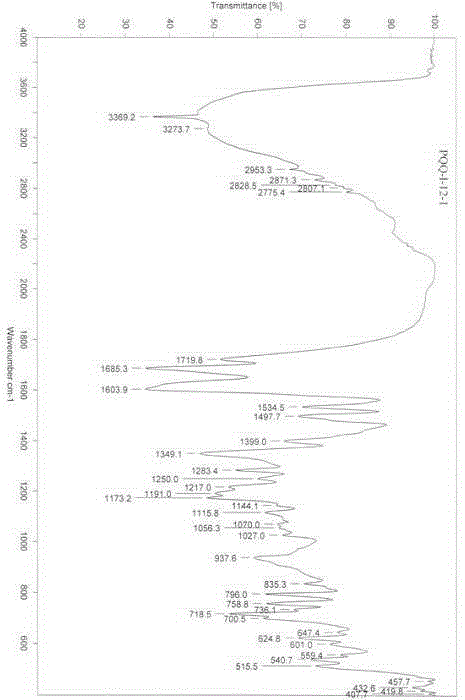
Pyrrolo quinoline quinone (PQQ) disodium salt impurity separation and purification method
ActiveCN105334301AEfficient separationPreparing sample for investigationTesting medicinal preparationsRedox enzymesChemical structure
The invention provides a pyrrolo quinoline quinone (PQQ) disodium salt impurity separation and purification method, belonging to the technical field of chemical drug impurity research. PQQ is a prothetic group of multiple oxidation-reduction enzymes, has remarkable effects in preventing and treating alcoholic fatty liver, is expected to become a novel liver injury prevention and cure drug, and has good clinical application prospect. Aiming at the impurity in the drug, quality control limit must be established according to the physiological activity itself; the method comprises effectively separating the impurity in the drug, thus ensuring drug quality and reducing untoward effects of the drug. Impurity separation and purification is the most key technology in drug research. PQQ synthesis by-product impurities are effectively separated to prepare a 100-mg impurity product, and high resolution mass spectrum (HRMS), infrared absorption spectrum (IR) and nuclear magnetic resonance spectrum (NMR) are adopted to confirm a chemical structural formula of the impurity and finally confirm a molecular formula, chemical construction and a chemical name of the impurity, thus providing a reliable basis for impurity limit formulation for reporting PQQ clinic medication.
Owner:WEIFANG SHENGYU PHARMA CO LTD
Preparation method of prostaglandin medicine impurity
ActiveCN103601708AMeet quality requirementsHigh yieldOrganic chemistryComponent separationBoron trichlorideKetone
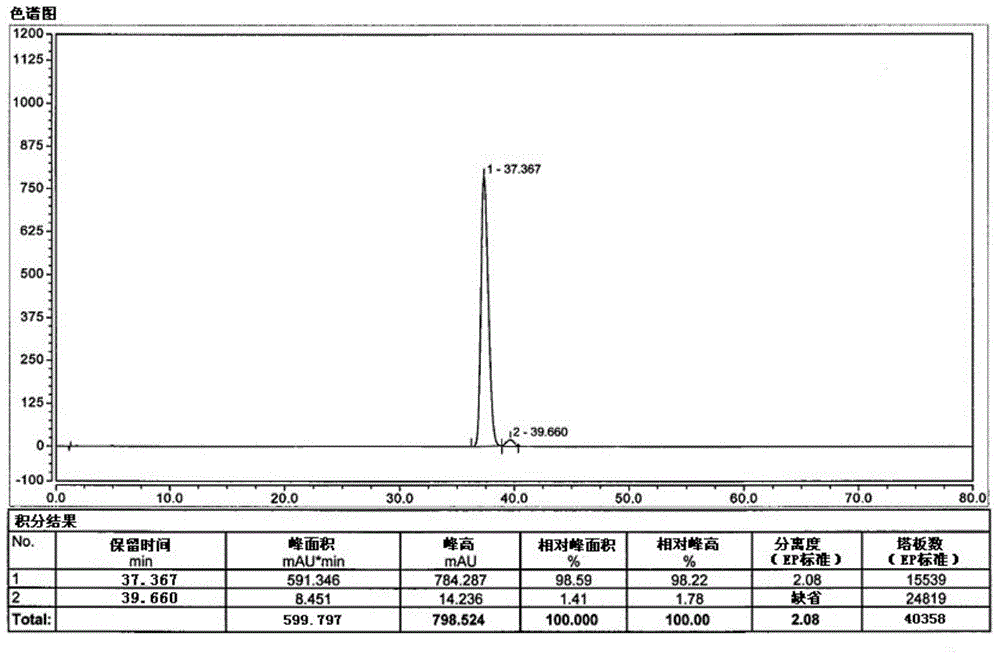
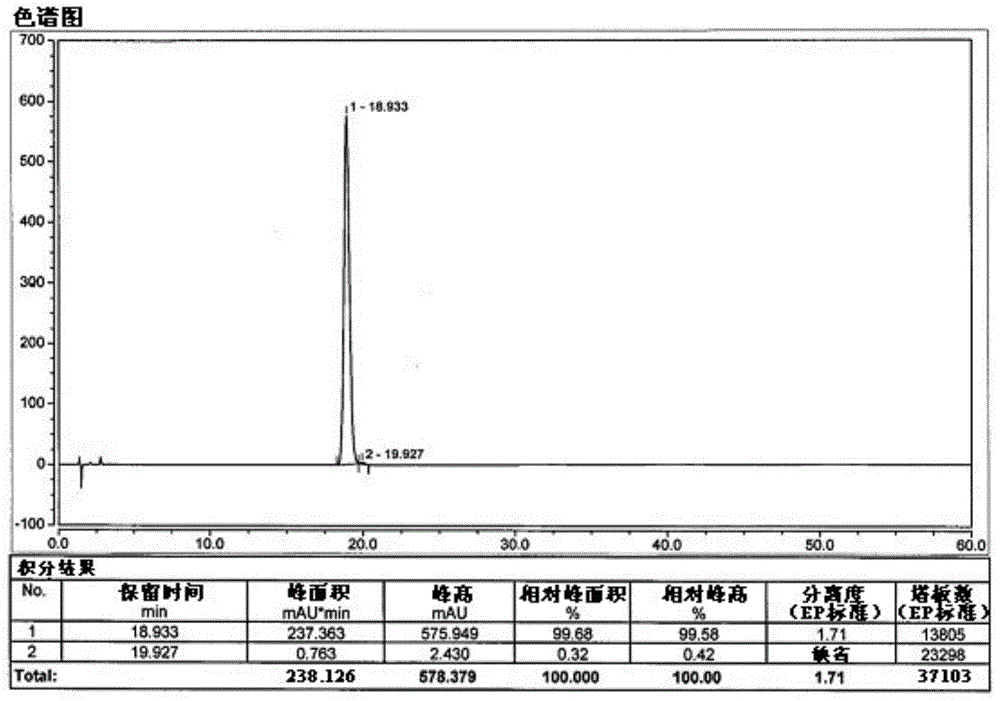
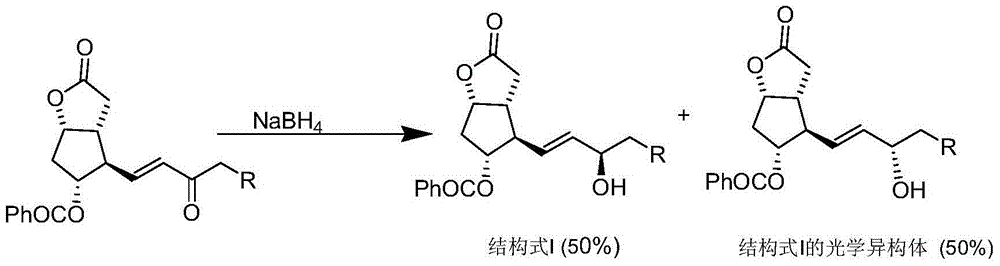
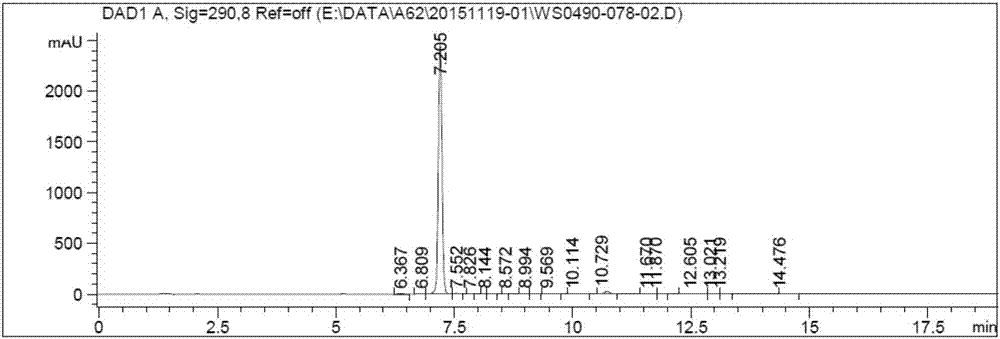
The invention belongs to the field of medicine synthesis, and particularly relates to a novel preparation method of a key impurity of a prostaglandin series compound. The preparation method is characterized in that (-)alpha-pinene, sodium borohydride and boron trichloride are used as raw materials, firstly a chiral reducing agent is prepared, and then a midbody of the prostaglandin series compound, namely precursor ketone is subjected to chiral catalytic reduction, thus the target impurity is obtained. The preparation method provided by the invention has the advantages that the synthesis of the impurity is simple, convenient and feasible, the yield is high, and the optical purity is good; a relatively good impurity reference substance is supplied for the quality research and the quantitative control on the impurity of industrially produced prostaglandin series products; the figure 1 is a HPLC (High Performance Liquid Chromatography) map of the impurity A'.
Owner:WUHAN WUYAO SCI & TECH
Canagliflozin drug impurity as well as preparation method and application thereof
ActiveCN107286143AStarting materials are cheap and readily availableReduce stepsOrganic chemistry methodsOrganic solventLithium hydroxide
The invention discloses a canagliflozin drug impurity as well as a preparation method and application thereof. The invention provides a compound as well as a preparation method and application thereof. The method comprises the following steps: (1) enabling the compound as shown in formula 2 to be in contact with an alkaline lithium hydroxide aqueous solution to obtain a coarse product containing a compound as shown in formula 3, wherein the coarse product contains a compound as shown in formula 1; (2) crystallizing and filtering the coarse product to obtain mother liquor; (3) concentrating the mother liquor to obtain residues; and (4) crystallizing and filtering the residues in an L-proline-containing organic solvent, thus obtaining the compound as shown in formula 1. The method provided by the invention can realize directed preparation of the compound as shown in formula 1, and a reliable impurity contrast is provided for quality research on industrially produced canagliflozin-series diabetes treatment drug products and quantitative control over impurities.
Owner:WATERSTONE PHARMA WUHAN
Delafloxacin impurity IV and product refining method
InactiveCN111718329AControl the risk of security incidentsThe refining method is simpleComponent separationOrganic compound preparationMedicinePhysical chemistry
The invention belongs to the field of medicine impurities, and particularly relates to a delafloxacin impurity IV and a product refining method. A proper analysis method is selected through optimization to detect related stubborn impurities of a product. The specific structure of related impurities is separated, detected and confirmed. Finally, synthesis and process routes are optimized, impurities are controlled within a safe range, a basis is provided for product process amplification, stability, quality, pharmacology and toxicology and clinical research, and the method has important significance in reducing the safety risk of drug impurities.
Owner:NANJING HAIRUN PHARM CO LTD +1
Synthesizing method of ambroxol hydrochloride related substance
PendingCN109810066AReduce processing difficultyLow costOrganic chemistryOrganic synthesisDrug Impurity
The invention discloses a synthesizing method of an ambroxol hydrochloride related substance, and relates to the technical field of organic synthesis. Ambroxol hydrochloride serves as a raw material,is subjected to a cyclization reaction with formaldehyde firstly to synthesize 4-(6,8-dibromo-1,4-dihydroquinazolin-3(2H)-yl)-cyclohexanol hydrochloride, and then is subjected to an oxidation reactionto synthesize 4-(6,8-dibromo-3,4-dihydroquinazolin-3-yl)-cyclohexanol hydrochloride. According to the synthesizing method, the product yield can reach 90% or above, meanwhile the purity reaches 99% or above, the requirement for serving as a drug impurity standard product is met, and thus the content of the impurity 4-(6,8-dibromo-3,4-dihydroquinazolin-3-yl)-cyclohexanol hydrochloride in the ambroxol hydrochloride is controlled advantageously.
Owner:HEFEI UNIV
Preparation method of chlorpheniramine maleate impurity
The invention provides a preparation method of a chlorpheniramine maleate impurity, and belongs to the technical field of preparation of drug impurity standard substances. The method comprises the following steps: dissolving a compound shown in a formula 1 in a first solvent, conducting reacting with a first alkali for 30 minutes at a first temperature, and then conducting reacting with a compound shown in a formula 2 to obtain a compound shown in a formula 3; 2, reacting the compound shown in the formula 3 with second alkali in a second solvent at a second temperature to obtain a compound shown in a formula 4; and 3, reacting the compound shown in the formula 4 with a third alkali in a third solvent at a third temperature for 30 minutes, and conducting reacting with a compound shown in a formula 5 to obtain the chlorpheniramine maleate impurity. The method for preparing the chlorpheniramine maleate impurity has the advantages of simplicity in operation, short preparation period, few byproducts, easiness in purification and high yield, solves the problem of shortage of reference substances, and provides standard reference substances for quality control of chlorpheniramine maleate raw material medicines and finished preparation products.
Owner:艾希尔(深圳)药物研发有限公司
Control method of piperaquine phosphate impurity
ActiveCN104402815ASimple processWide variety of sourcesOrganic chemistryComponent separationActivated carbonReflux
The invention relates to the field of piperaquine phosphate impurity control, and specifically relates to a control method of piperaquine phosphate impurity. The control method of piperaquine phosphate impurity comprises the following steps: 1), adding a non-polar solvent into a piperaquine phosphate crude product, stirring and filtering; 2), adding a polar solvent, conducting reflux, stirring and filtering; 3), adding a water / polar mixed solvent, stirring and dissolving at 90-110 DEG, adding activated carbon for decoloring and filtering to obtain a filtrate, cooling, crystallizing, filtering, washing and drying. The steps 1) 2) and 3) are not in a particular order. The control method of piperaquine phosphate impurity provided by the invention has the advantages of simple process, and extensive equipment and reagent sources, thus indirectly reducing cost for drug impurity removal, and can be widely applied to preparation of piperaquine phosphate.
Owner:GUILIN PHARMA
GMDTC drug impurity and preparation method thereof
PendingCN113845489ANovel structureSimple manufacturing methodOrganic chemistryComponent separationCombinatorial chemistryDrug Impurity
The invention discloses a GMDTC drug impurity. The structure of the GMDTC drug impurity is shown as a formula (I). The invention further provides a preparation method of the GMDTC medicine impurity; the impurity can be used as an impurity standard substance or reference substance in GMDTC raw material medicine or preparation detection, and the quality of a GMDTC preparation is improved, so that the use safety and effectiveness of the GMDTC preparation are ensured.
Owner:健尔圣(珠海)医药科技有限公司
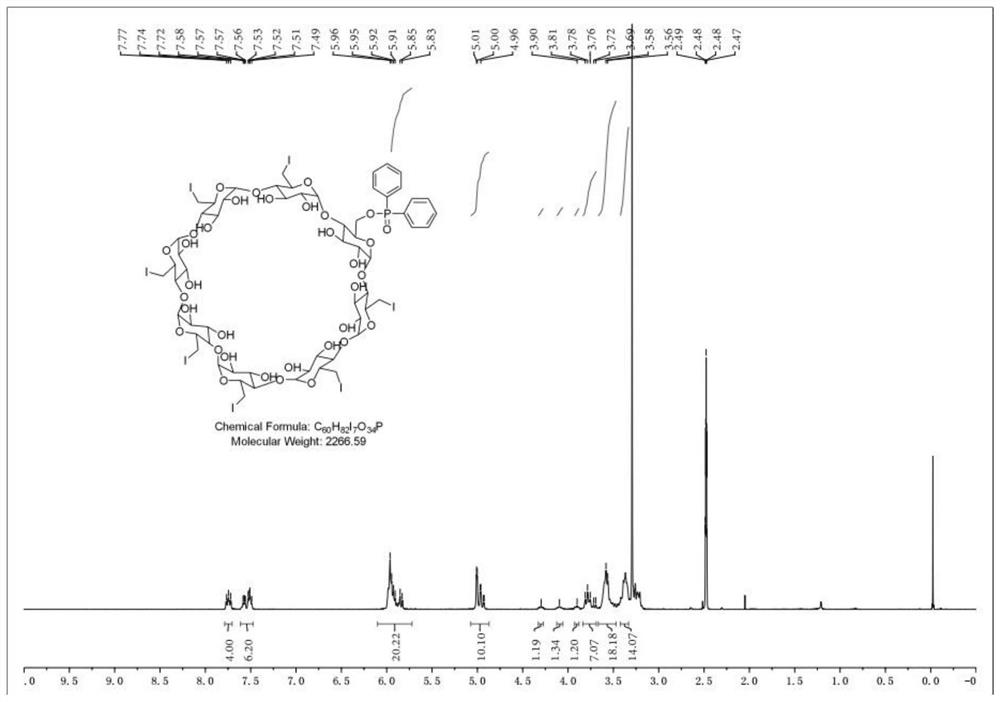
Sugammadex sodium intermediate diphenylphosphinic acid derivative impurity and preparation method thereof
The invention relates to the technical field of medicine impurity synthesis. The invention discloses a diphenylphosphinic acid derivative impurity in a sugammadex sodium intermediate gamma-ICD and a preparation method of the diphenylphosphinic acid derivative impurity. The preparation method of the impurity comprises the following steps: (1) dissolving diphenylphosphinic acid in an organic solvent, adding a certain amount of alkali under the protection of inert gas, carrying out stirring and reacting for a period of time at a certain temperature, then adding 6-total deoxy-6-total iodo-gamma-cyclodextrin, reacting for a period of time at a certain temperature, and carrying out aftertreatment to obtain a compound crude product shown as a formula I; and (2) separating and purifying the crudeproduct to obtain the single compound shown in the formula I. The invention provides a standard reference substance for quality control of the sugammadex sodium intermediate gamma-ICD, and is particularly important for impurity research and quality control of the sugammadex sodium intermediate gamma-ICD; and the preparation method disclosed by the invention has the beneficial effects that the operation is convenient, the reaction conditions are mild and controllable, the reaction stability is high, and the reaction product is high in yield and purity.
Owner:武汉嘉诺康医药技术有限公司
Preparation method for N-nitrosamine genotoxic impurity of varenicline tartrate
The invention provides a preparation method for an N-nitrosamine genotoxic impurity of varenicline tartrate. The preparation method comprises the following steps: with ammonium formate as a hydrogen donor, carrying out reduction reaction on dinitrate under the catalysis of palladium carbon to obtain a diamino compound, carrying out a cyclization reaction on the diamino compound and an aqueous glyoxal solution to obtain a cyclization product, carrying out a hydrolysis reaction on the cyclization product under the action of sodium hydroxide, removing trifluoroacetyl to obtain free alkali, and finally, subjecting the free alkali to reacting with sodium nitrite under the catalysis of protonic acid to obtain the N-nitrosamine impurity. According to the invention, the technical vacancy of preparation methods for the impurity at present is filled in, the prepared high-purity impurity can be applied as a control sample to the drug impurity research and production quality control process of varenicline tartrate, and a guarantee is provided for the genotoxicity research and comprehensive quality control of a varenicline tartrate bulk drug.
Owner:JIANGSU SINOBIOPHARMA
Method for treating sewage of pharmaceutical factory
InactiveCN110627277AAvoid pollutionWater/sewage treatment by irradiationMultistage water/sewage treatmentFiltrationDecomposition
The invention discloses a method for treating sewage of a pharmaceutical factory. The method comprises the following steps: introducing sewage into a primary filtering tank for preliminary filtration,introducing the sewage into a dust sand tank, adding a flocculating agent with a mass-volume ratio of (120-180g):1L to the sewage, carrying out stirring for 5-15 min, then carrying out stirring for 30-40 min at a rotating speed of 150-250 r / min, introducing sewage into an aeration tank, and introducing oxygen into the aeration tank; introducing the sewage in the aeration tank into a photoreactor,adding H2O2 with a mass content of 20%, carrying out irradiating with ultraviolet light, and carrying out stirring for 20-30 min; adding Na2SO3 with a molar concentration of 0.1 mM until no bubbles are generated in the sewage; and analyzing the result, and sending the sewage into a contact disinfection tank to react the sewage with ClO2 for disinfection, and discharging the effluent of the disinfection tank into a common sewage treatment tank. The method provided by the invention can be used to carry out oxidative decomposition on drug impurities in the wastewater of the pharmaceutical factory, pre-treat drug wastewater and prevent environmental pollution.
Owner:常飞
Dipeptide for synthesizing relin drugs
ActiveCN109293736AReduce generationHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsDipeptideL-Pyroglutamic Acid
The invention provides a dipeptide having the structure of formula I and a preparation method thereof. Pyroglutamic acid (Pyr) and histidine (His) can be introduced into relin peptide-like drugs by solid-phase synthesis in one step by using dipeptide segments, which greatly reduces the production of [D-His<2>] relin drug impurity, improves the yield and purity of the product, and the reaction efficiency is high, which is conducive to the realization of large-scale solid-state synthesis process.
Owner:QILU PHARMA CO LTD
Delafloxacin impurities I and II and product refining method
InactiveCN111718331AControl the risk of security incidentsThe refining method is simpleOrganic chemistryMedicineBiochemical engineering
The invention belongs to the field of medicine impurities, and particularly relates to delafloxacin impurities I and II and a product refining method. A proper analysis method is selected through optimization to detect related stubborn impurities of a product. The specific structure of related impurities is separated, detected and confirmed. Finally, synthesis and process routes are optimized, impurities are controlled within a safe range, a basis is provided for product process amplification, stability, quality, pharmacology and toxicology and clinical research, and the method has important significance in reducing the safety risk of drug impurities.
Owner:NANJING HAIRUN PHARM CO LTD +1
Preparation method of calcium levofolinate impurity and impurity calcium salt
ActiveCN110229155ASimple processLow equipment requirementsOrganic chemistryCalcium levofolinateDihydrofolic acid
Belonging to the field of chemical pharmacy, the invention relates to a preparation method of a standard drug impurity substance, in particular to the preparation method of a calcium levofolinate impurity and an impurity calcium salt. Firstly, the invention provides a preparation method of calcium levofolinate impurity, and the method includes: taking the compound folic acid shown as formula I asthe raw material for reaction with a reductant in an aqueous solution, and adjusting the pH value of the reaction solution with an alkali aqueous solution and inorganic acid respectively to obtain thecompound dihydrofolic acid shown as formula II; and reacting the compound shown as II with formic acid, adding purified water, and performing stirring and filtering to obtain the compound 10-formyl dihydrofolate shown as formula III. The invention also provides a preparation method of 10-calcium formyl dihydrofolate, and the method includes: taking folic acid as the raw material, firstly preparing dihydrofolic acid, then reacting dihydrofolic acid with formic acid to obtain 10-formyl dihydrofolate, then adding a calcium salt, and carrying out reaction to obtain 10-calcium formyl dihydrofolate.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Cefpodoxime proxetil impurity cefpodoxime dipivoxil and preparation method thereof
The invention relates to a cefpodoxime proxetil impurity, namely cefpodoxime dipivoxil and a preparation method thereof, belonging to the technical field of drug impurity synthesis. The invention aims to provide a novel impurity with high purity and quality. According to the invention, the method comprises the following steps: adding raw materials including cefpodoxime proxetil and 1-iodoethyl isopropyl carbonate into a non-water-soluble benign organic solvent, adding organic alkali to control the pH value of the system to be 8.0-10, carrying out condensation reaction under the condition that a temperature is controlled to be 20-45 DEG C, and obtaining the corresponding product cefpodoxime proxetil impurity, namely cefpodoxime dipivoxil after the reaction is ended. The novel impurity provided by the invention can be used for impurity control of cefpodoxime proxetil synthesis, and has the advantages of high yield and product purity of 93% or more.
Owner:浙江东邦药业有限公司
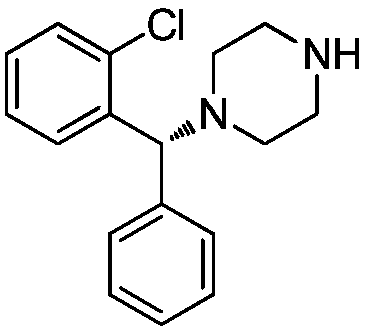
A kind of resolution method of (r)-1-((2-chlorophenyl)-(phenyl)-methyl)-piperazine
ActiveCN105924409BHigh purityReduce pollutionOptically-active compound separationOrganic racemisationAlpha-methylbenzyl isocyanateReaction temperature
The invention belongs to the technical field of chiral drug impurity preparation, and mainly relates to a resolution method of a chiral drug levocetirizine hydrochloride impurity (R)-1-((2-chlorphenyl)-(phenyl)-methyl)-piperazine. The resolution method comprises the steps that racemic 1-((2-chlorphenyl)-(phenyl)-methyl)-piperazine and a resolution agent (S)-(-)-alpha-methylbenzyl isocyanate are dissolved into an organic solvent and react to generate precipitate of (R)-1-((2-chlorphenyl)-(phenyl)-methyl)-piperazine-(S)-(-)-alpha-methylbenzyl isocyanate salt, the precipitate is filtered and washed with acetone to obtain the (R)-1-((2-chlorphenyl)-(phenyl)-methyl)-piperazine-(S)-(-)-alpha-methylbenzyl isocyanate salt, the (R)-1-((2-chlorphenyl)-(phenyl)-methyl)-piperazine-(S)-(-)-alpha-methylbenzyl isocyanate salt is dissolved by adding water, the pH is regulated with a saturated sodium carbonate solution to be 8.0, extraction is conducted by adding ethyl acetate, and drying, filtering and concentrating are conducted to obtain (R)-1-((2-chlorphenyl)-(phenyl)-methyl)-piperazine. The resolution method has the advantages that the product purity is high, the reaction time is short, the reaction temperature is low, and the yield is high; in addition, the solvents are acetone and ethyl acetate and are easy to obtain, free of toxicity, easy to recycle and little in environmental pollution.
Owner:ZHEJIANG YONGNING PHARMA
Preparation method of high-purity amino sugar drug impurity
The invention discloses a preparation method of a high-purity amino sugar drug impurity, the amino sugar drug impurity has a structure as shown in formula (I): weak acid is used for catalyzing valiolamine to react with 1, 3-dihydroxy acetone to prepare the amino sugar drug impurity, and the HPLC (high performance liquid chromatography) purity in a reaction product is greater than 20%. The method is high in yield, environment-friendly and suitable for industrial production of amino sugar drug impurities. The invention further discloses a method for purifying the amino sugar drug impurities from the reaction liquid, and the optimal HPLC purity of the purified amino sugar drug impurities is 98% or above.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
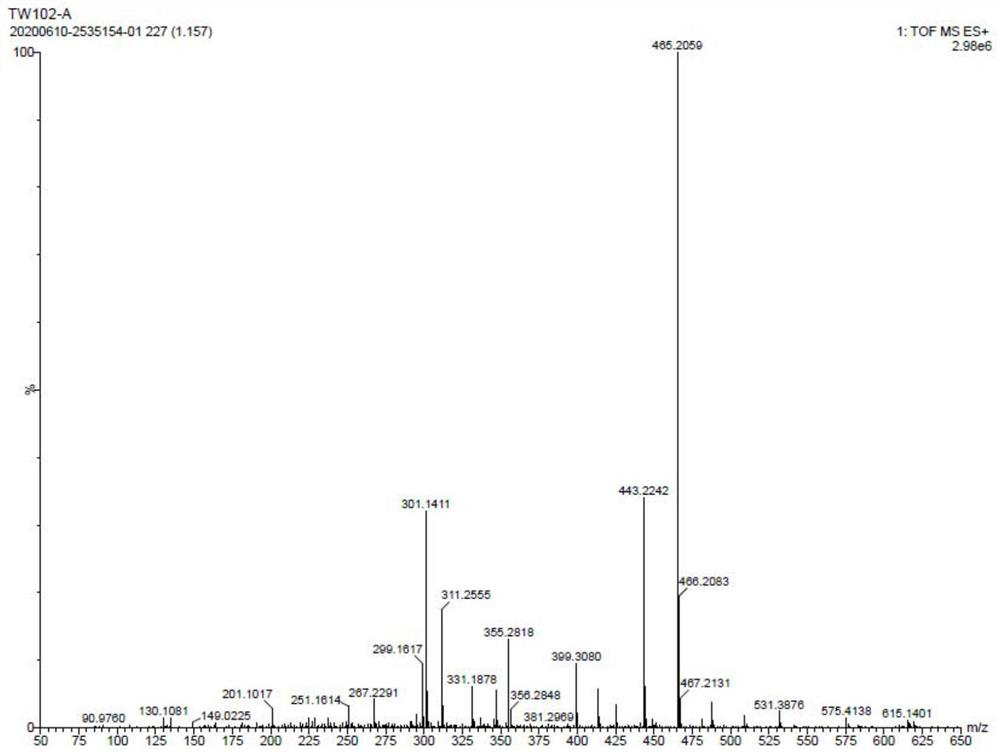
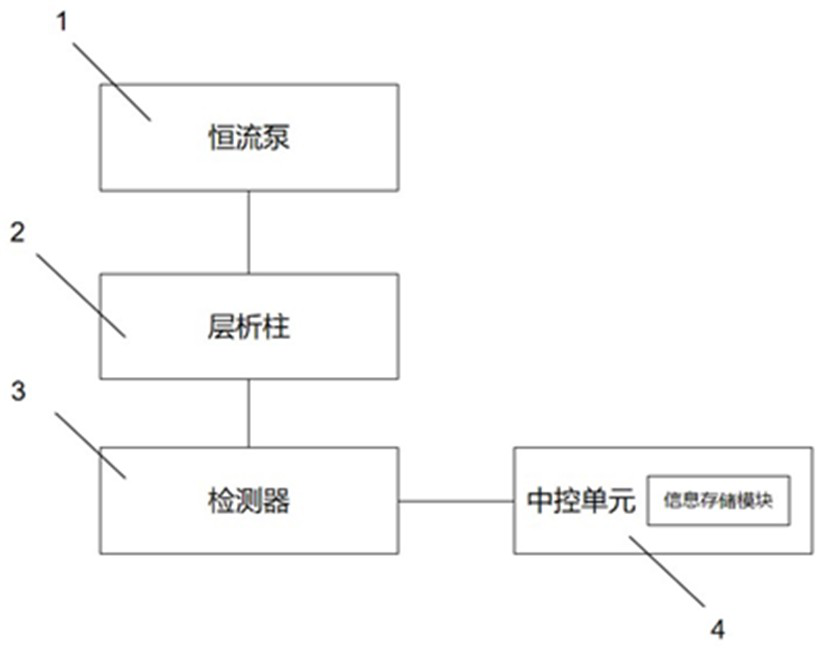
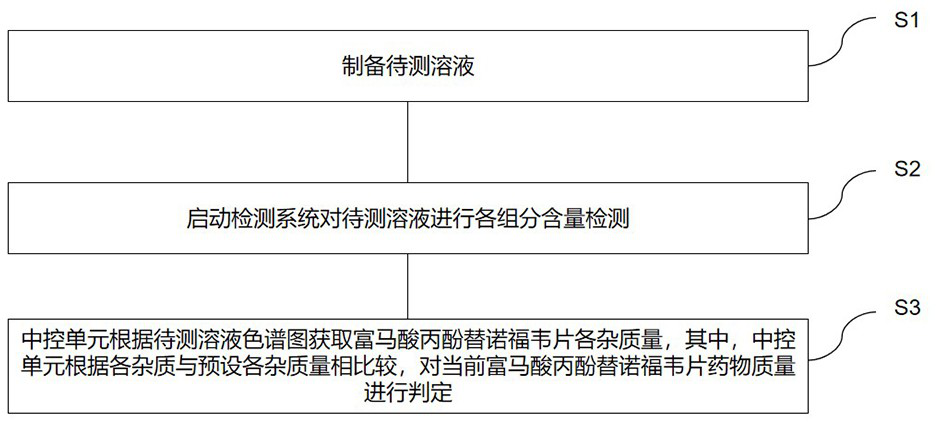
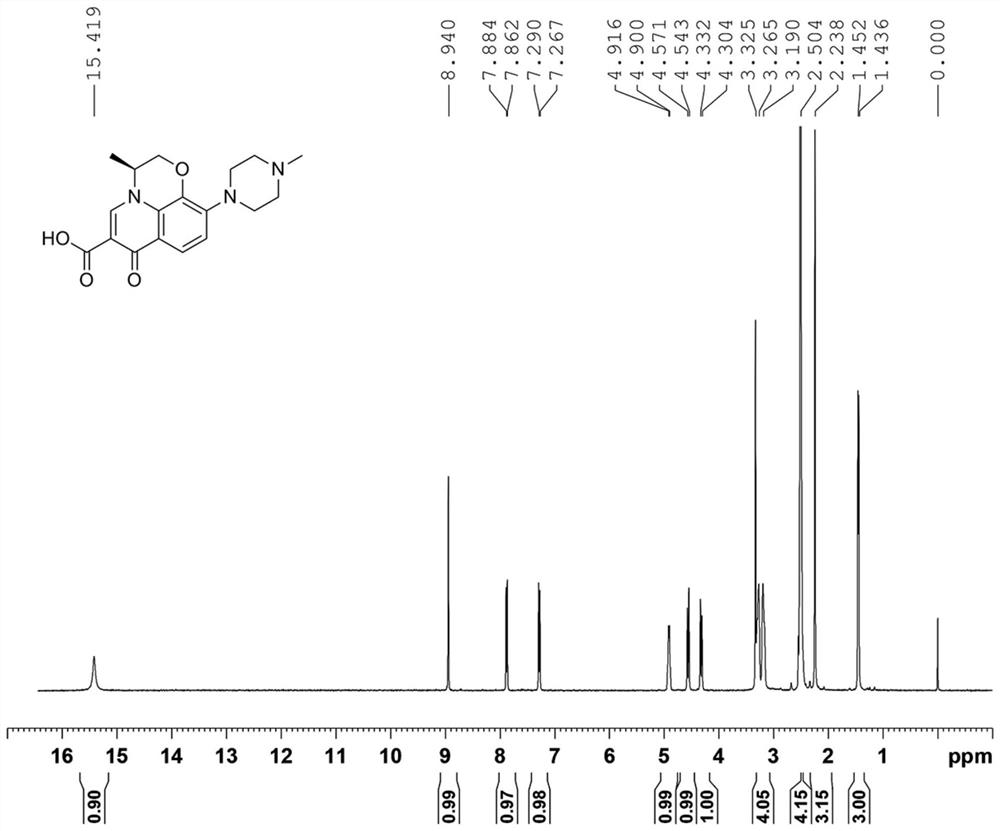
Drug impurity detection method based on tenofovir alafenamide fumarate tablets
ActiveCN114295767BEasy to cleanSolve the problem of more dirtComponent separationPharmaceutical drugElution
The invention relates to a drug impurity detection method based on tenofovir alafenamide fumarate tablets, comprising, step S1, preparing a solution to be tested, the solution to be tested includes a sensitivity solution, a control solution, a system adaptability solution and a sample solution; Step S2, start the detection system to detect the content of each component in the solution to be tested. If the sensitivity of the current detection system does not meet the preset standard, the central control unit will store the last detection result in the information storage module to perform a test on the gradients of each stage set in the detection system. Adjust the phase content and elution time, start the cleaning device installed in the chromatography column, and the central control unit obtains the cleaning frequency to clean the chromatography column according to the last detection result stored in the information storage module; step S3, the central control unit cleans the chromatography column according to the Measure the chromatogram of the solution to obtain the amount of each impurity in tenofovir alafenamide fumarate tablets. Among them, the central control unit compares the amount of each impurity with the preset amount of impurities to determine the quality of the current tenofovir alafenamide fumarate tablet. Make a judgment.
Owner:HUNAN MINGRUI PHARMA
Preparation method of levofloxacin defluorination impurity
PendingCN112174981AShort preparation cycleHigh purityOrganic chemistryPreparing sample for investigationPtru catalystPhysical chemistry
The invention provides a preparation method of a levofloxacin defluorination impurity, and belongs to the technical field of preparation of drug impurity standard substances, and the preparation method comprises the following steps: step 1, carrying out substitution reaction on a compound shown in a formula I and sodium methyl mercaptide in a first solvent at a first temperature to obtain a compound shown in a formula III; and 2, carrying out a reduction reaction on the compound represented by the formula III in the presence of hydrogen and a catalyst at a second solvent and a second temperature to obtain the levofloxacin defluorination impurity. According to the method, levofloxacin is used as a raw material, the method has the advantages of being simple to operate, short in preparation period, few in by-product, easy to purify, high in yield and environmentally friendly, and the prepared levofloxacin defluorination impurity is high in purity, meets the requirement of an impurity reference substance, can be used as a levofloxacin defluorination impurity standard substance for qualitative and quantitative research and detection of levofloxacin defluorination impurities.
Owner:深圳市祥根生物科技有限公司
Preparation method of acemetacin impurity D
The invention belongs to the technical field of drug impurity synthesis, and particularly relates to a preparation method of an acemetacin impurity D. The acemetacin impurity D is prepared by taking indometacin as a raw material through six-step reaction. The method has the advantages of easily available raw materials, simple operation, short linear route, few reaction steps, rapid preparation of acemetacin impurity D, and high purity; the preparation efficiency is improved, and the preparation cost is reduced.
Owner:BEIJING INSTITUTE OF CLOTHING TECHNOLOGY +1
Preparation method of pramipexole N methyl impurity
The invention provides a preparation method of a pramipexole N methyl impurity, and belongs to the technical field of preparation of drug impurity standard substances. The preparation method comprises the following steps: 1, enabling reaction of a compound as shown in a formula 1 with (Boc) 2O in a first solvent under the action of first alkali to obtain a compound as shown in a formula 2; and 2, enabling reaction of the compound shown in the formula 2 in a second solvent under the action of LAH (lithium aluminum hydride) to obtain the pramipexole N-methyl impurity. The invention aims to provide the preparation method of the pramipexole N-methyl impurity, which has the advantages of simplicity in operation, short preparation period, few byproducts, easiness in purification and high yield, solves the problem of shortage of reference substances, and provides standard reference substances for quality control of pramipexole bulk drugs and preparation finished products.
Owner:艾希尔(深圳)药物研发有限公司
Avanafil impurity D as well as synthesis method and application thereof
InactiveCN113880817APerfect drug impurity profileHigh purityOrganic chemistryCombinatorial chemistryDrug Impurity
The invention provides an avanafil impurity D as well as a synthesis method and application thereof. According to the novel avanafil impurity and the synthesis method thereof, the structure of the impurity is determined by synthesizing and characterizing the impurity, the drug impurity spectrum of avanafil is perfected, and the obtained product is high in purity and can be used as a reference substance for drug quality control research.
Owner:HARVEST PHARMA HUNAN CO LTD
Preparation method of ibuprofen impurity B
InactiveCN112479855AHigh purityEasy to operateOrganic compound preparationCarboxylic acid esters preparationPtru catalystEthyl acetate
The invention provides a preparation method of an ibuprofen impurity B, and belongs to the technical field of preparation of drug impurity standard substances, and the preparation method comprises thefollowing steps: step 1, carrying out coupling reaction on a compound shown in a formula 1 and ethyl bromoacetate under the action of a catalyst and a first alkali to obtain a compound shown in a formula 2; 2, performing substitution reaction on the compound shown in the formula 2 and methyl iodide under the action of second alkali to obtain a compound shown in a formula 3; and 3, hydrolyzing thecompound shown in the formula 3 under the action of a third alkali to obtain the ibuprofen impurity B. The method has the advantages of simplicity in operation, short preparation period, few byproducts, easiness in purification and high yield, the prepared ibuprofen impurity B has high purity and no obvious impurity point, meets the requirements of impurity reference substances, can be used as anibuprofen impurity B standard substance, is applied to qualitative and quantitative research and detection of the ibuprofen impurity B, and has certain significance for quality control of ibuprofen bulk drugs and related preparations thereof.
Owner:艾希尔(深圳)药物研发有限公司
Method for simply detecting content of medicine component
InactiveCN113219143ALow costGood reproducibilityPreparing sample for investigationTesting medicinal preparationsEngineeringDrug Impurity
The invention discloses a method for simply detecting the content of medicine components. The method comprises the following steps: S1, sample pretreatment: pretreating a sample according to detected requirements, firstly removing medicine impurities, purifying the sample, performing enrichment concentration and derivatization on the detected sample, enabling the form of the sample and the used solvent to meet the requirements of analysis and determination, and completing the pretreatment of a medicine; S2, sample crushing: crushing the pretreated medicine according to detected requirements, sieving the crushed medicines during crushing, enabling the medicines meeting the sieving requirements to pass through sieve pores in time, preventing the medicines from being crushed too fine, and continuously crushing the medicines which cannot pass through the sieve pores. The method for simply detecting the content of the medicine components is mainly characterized in that the method for detecting the content of the medicine components is low in instrument and equipment cost, simple, high in detection precision and accuracy, good in detection effect and high in efficiency.
Owner:广东恒锦通科技有限公司
A kind of environment-friendly preparation method of ibuprofen impurity h
ActiveCN111269100BHigh purityEasy to operateOrganic compound preparationComponent separationChemical compoundDrug Impurity
The invention provides an environment-friendly preparation method of an ibuprofen impurity H, and belongs to the technical field of preparation of drug impurity standard substances. The method comprises the following steps: step 1, reacting a compound shown as a formula (I) with a compound shown as a formula (II) under the catalytic action of NaOC2H5 to prepare a compound shown as a formula (III);and step 2, reacting the compound shown in the formula (III) in the presence of CH3MgBr to prepare the ibuprofen impurity H. The method has the advantages of simplicity in operation, short preparation period, few byproducts, easiness in purification, high yield and environmental friendliness; the prepared ibuprofen impurity H is high in purity and free of obvious impurity points and meets the requirements of impurity reference substances and can be used as an ibuprofen impurity H standard substance to be applied to qualitative and quantitative research and detection of the ibuprofen impurityH so as to improve the accuracy of the detection method.
Owner:深圳市祥根生物科技有限公司
The control method of piperaquine phosphate impurity
ActiveCN104402815BSimple processWide variety of sourcesOrganic chemistryComponent separationRefluxActivated carbon
The invention relates to the field of piperaquine phosphate impurity control, and specifically relates to a control method of piperaquine phosphate impurity. The control method of piperaquine phosphate impurity comprises the following steps: 1), adding a non-polar solvent into a piperaquine phosphate crude product, stirring and filtering; 2), adding a polar solvent, conducting reflux, stirring and filtering; 3), adding a water / polar mixed solvent, stirring and dissolving at 90-110 DEG, adding activated carbon for decoloring and filtering to obtain a filtrate, cooling, crystallizing, filtering, washing and drying. The steps 1) 2) and 3) are not in a particular order. The control method of piperaquine phosphate impurity provided by the invention has the advantages of simple process, and extensive equipment and reagent sources, thus indirectly reducing cost for drug impurity removal, and can be widely applied to preparation of piperaquine phosphate.
Owner:GUILIN PHARMA
Preparation method of phenylephrine hydrochloride impurity standard substance
ActiveCN111606815AFulfil requirementsHigh yieldOrganic compound preparationAmino-hyroxy compound preparationNitromethanePharmaceutical Substances
The invention provides a preparation method of a phenylephrine hydrochloride impurity standard substance, which comprises the following steps: putting an phenylephrine hydrochloride bulk drug under anillumination condition to generate impurities, and obtaining chromatographically pure target impurities by preparative chromatography; in the presence of ketoreductase KRED and reduced coenzyme II NADPH, taking nitromethane and m-hydroxybenzaldehyde as raw materials to react to obtain a concentrate; and finally, recrystallizing the concentrate by using an isopropanol-n-propyl acetate-acetonitrilemixed solution, adding chromatographically pure target impurities neutralized by alkali in the recrystallization process, and salifying to obtain the phenylephrine hydrochloride impurity standard substance (impurity A) which is high in product yield and purity and meets the requirements of drug impurity standard substances.
Owner:珠海安哲生物科技有限公司
Urapidil impurity compound, preparation method and application thereof
PendingCN112094239AHigh purityAvoid product qualityOrganic chemistryComponent separationOrganic solventChemical compound
The invention belongs to the technical field of medicine impurity preparation, and discloses an urapidil impurity compound, a preparation method and application thereof. The preparation method of theurapidil impurity compound comprises the following steps that 1), urapidil is dissolved in a mixed solvent of an organic solvent and water, and after heating dissolution is completed, carbon dioxide is introduced for a pressurization reaction; and 2) after the reaction is finished, the reaction solution isconcentrated and dried under reduced pressure, and purifying is carried out by column chromatography to obtain the urapidil impurity compound. The impurity compound meets the requirements of an impurity reference substance in quality control, can be used for quality control in a urapidil or urapidil hydrochloride synthesis process, can be used as the impurity reference substance for accurate quantitative detection of urapidil or urapidil hydrochloride, and is beneficial to improvement ofquality control of corresponding bulk drugs.
Owner:燃点(南京)生物医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com