Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Piperaquine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
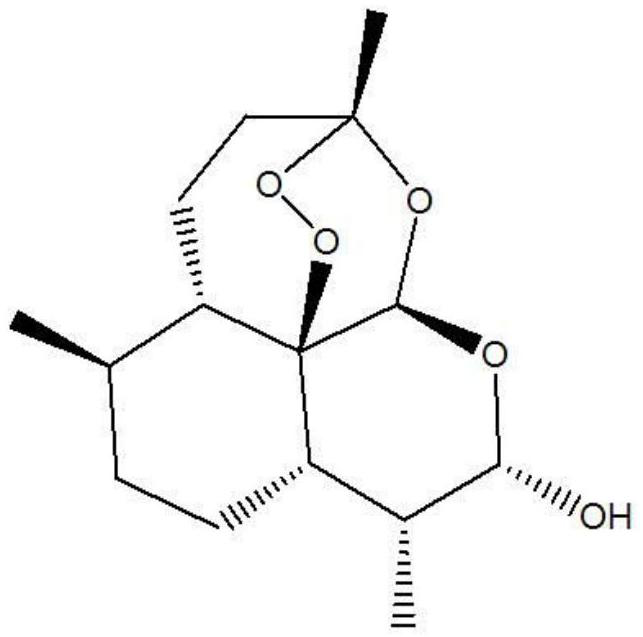
Piperaquine is an antiparasitic drug used in combination with dihydroartemisinin to treat malaria. Piperaquine was developed under the Chinese National Malaria Elimination Programme in the 1960s and was adopted throughout China as a replacement for the structurually similar antimalarial drug chloroquine. Due to widespread parasite resistance to piperaquine, the drug fell out of use as a monotherapy, and is instead used as a partner drug for artemisinin combination therapy. Piperaquine kills parasites by disrupting the detoxification of host heme.
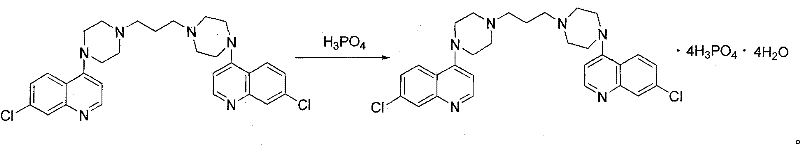
Preparation of piperaquini phosphatis
The invention discloses a method for preparing piperaquine phosphate. 4,7-dichloroquinoline is taken as an initial raw material and is subjected to condensation reaction with anhydrous piperazidine first to obtain 7-chloro-4-(1-piperazinyl)quinoline, then the 7-chloro-4-(1-piperazinyl)quinoline is subjected to condensation reaction with 1, 3-bromochloropropane to obtain piperaquine, and finally the piperaquine is salified with phosphorous acid to obtain the piperaquine phosphate. The method has a simple process, is easy to purify intermediate products and a final product, has high product yield and good quality, avoids the use of toxic reagents, has small pollution to the environment and low production cost, and is suitable for industrialized production.
Owner:CHONGQING KANGLE PHARMA
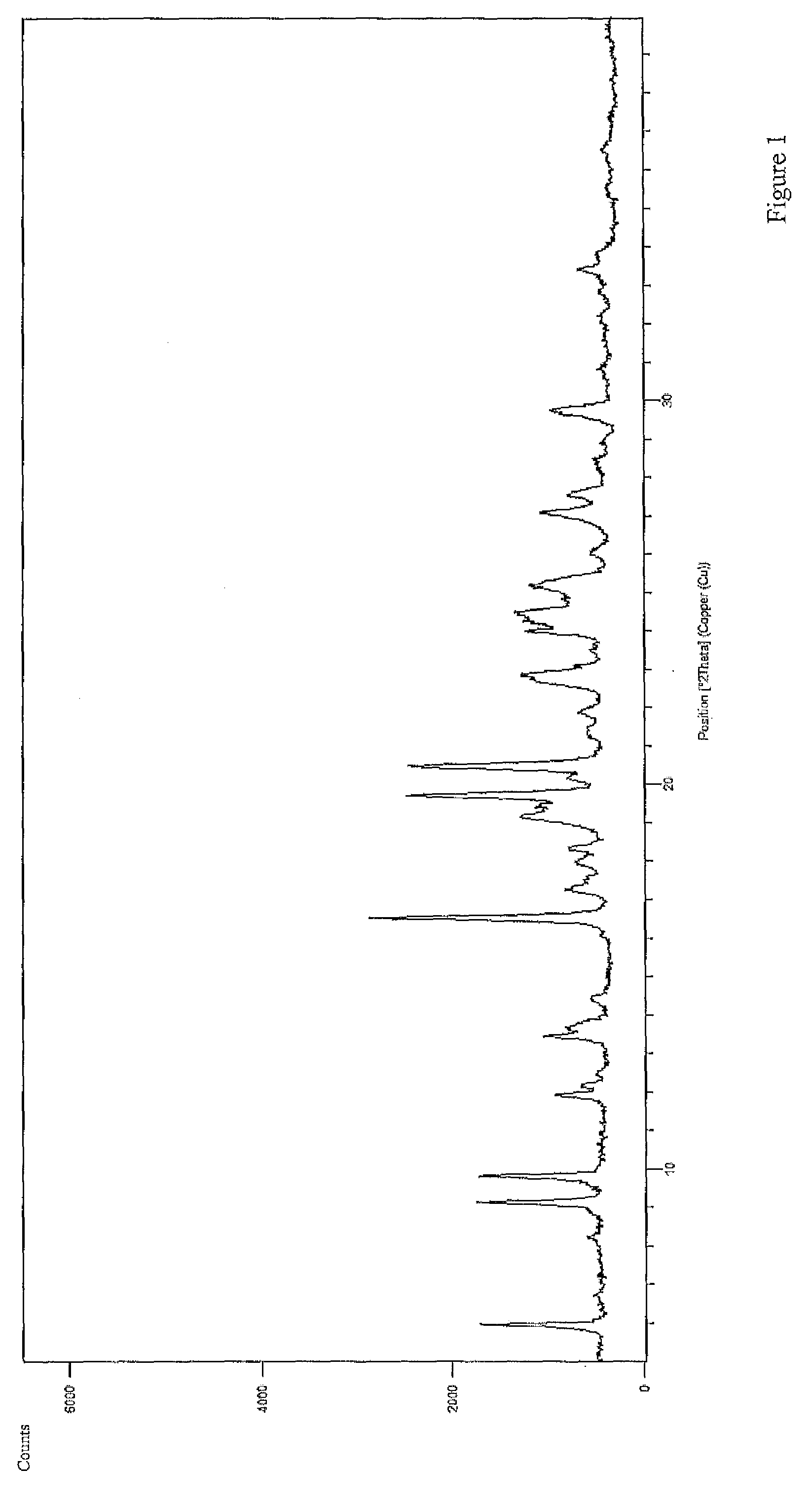
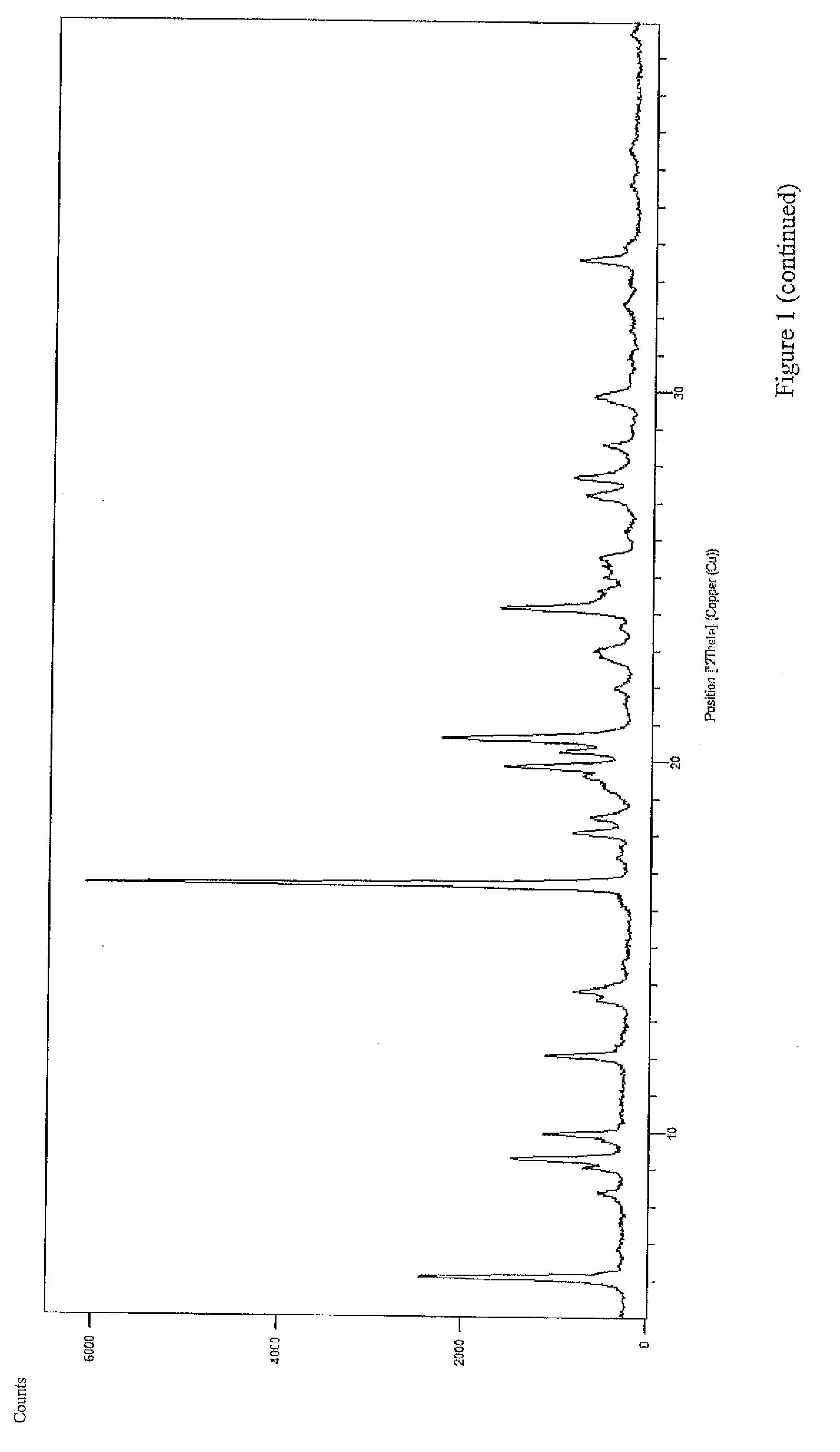
Polycrystal substance of piperaquine phosphate and preparation method thereof
ActiveCN102558048ALow toxicityProcess parameters are easy to controlOrganic chemistryPhosphateDissolution
The invention discloses a novel polycrystal substance of piperaquine phosphate. Compared with amorphous piperaquine phosphate, the polycrystal substance of the piperaquine phosphate, which is disclosed by the invention, has better stability and better facilitates the production, the storage and the circulation of raw medicine and preparations thereof, wherein a crystal form A of the piperaquine phosphate has the best stability, the best dissolution rate and the best dissolubility. The invention further discloses a preparation method of the polycrystal substance of the piperaquine phosphate. The method has the advantages of low toxicity, people friendliness, environment friendliness, easily-controlled technological parameters, and the like, is simple to operate and is suitable for mass production.
Owner:珠海润都制药股份有限公司
Dihydroartemisinin piperaquine phosphate tablets and preparation process thereof
InactiveCN101984970ASolve discolorationOne-sided bright and tidyOrganic active ingredientsAntiparasitic agentsPhosphateDihydroartemisinin
The invention discloses dihydroartemisinin piperaquine phosphate tablets. The dihydroartemisinin piperaquine phosphate tablets comprise the following raw materials in part by weight: 40 parts of dihydroartemisinin, 320 parts of piperaquine phosphate, 60 to 90 parts of starch, 10 to 25 parts of dextrin, 2 to 8 parts of hydroxypropyl methylcellulose, 20 to 80 parts of sodium carboxy-methyl starch, 2.5 to 3.0 parts of magnesium stearate and 12 to 18 parts of opadry. The invention also discloses a preparation method for the dihydroartemisinin piperaquine phosphate tablets. The preparation method comprises the following steps of: preparing materials, crushing, sieving, preparing a bonding agent, mixing and pelleting, drying, mixing and tabletting, and coating. The dihydroartemisinin piperaquine phosphate tablets have good stability and long storage time and are difficult to discolor.
Owner:贝克诺顿(浙江)制药有限公司
Compound artemisinin
The present invention provides a novel combination comprising artemisinin in the form of tablets and related dosage forms for pediatric use, such as granules, suppository, suspension syrup and dry powder, for the treatment of human malarias including multiple-resistant subtertian malaria, tertian malaria and quartan malaria. Said combination is comprised of artemisinin, piperaquine and primaquine. Clinical tests in Southeast Asia countries where malaria is epidemic demonstrate that, apart from having high and rapid therapeutic effect possessed by the most excellent domestic and foreign artemisinin-type anti-malarial drugs, the present combination is also featured with shorter course of treatment, less side effect, lower material cost, and more convenience for administration, and its ability of rapidly killing gametophyte and cutting off infection source thereby blocking spreading of malaria is a further improvement.
Owner:ARTEPHARM CO LTD CHINA
Double hydrogen arteannuic plain guagui slice and preparing method thereof
The invention discloses a piperaquine dihydroartemisinin tablet and the related preparation method, aiming at providing a piperaquine dihydroartemisinin tablet which is convenient to take and is prepared with dihydroartemisinin and piperaquine phosphate. The invention is made of 40 portions of dihydroartemisinin, 320 portions of piperaquine phosphate, 70 to 90 portions of starch, 20 to 30 portions of dextrin, 30 to 35 portions of sodium carboxymethyl starch, 6 to 10 portions of lhydroxypropyl cellulose and 3 to 5 portions of magnesium stearate (based on weight). The invention has the advantages of high finished product rate and low cost.
Owner:ZHUHAI HUAAO IMPORT & EXPORT CORP
Composition containing artemisinin for treatment of malaria
The present invention provides a novel combination comprising artemisinin in the form of tablets and related dosage forms for pediatric use, such as granules, suppository, suspension syrup and dry powder, for the treatment of human malarias including multiple-resistant subtertian malaria, tertian malaria and quartan malaria. The combination is comprised of artemisinin, piperaquine and primaquine. Clinical tests in Southeast Asia countries where malaria is epidemic demonstrate that, apart from having high and rapid therapeutic effect possessed by the most excellent domestic and foreign artemisinin-type anti-malarial drugs, the present combination is also featured with shorter course of treatment, less side effect, lower material cost, and more convenience for administration, and its ability of rapidly killing gametophyte and cutting off infection source thereby blocking spreading of malaria is a further improvement.
Owner:ARTEPHARM CO LTD CHINA
Preparation of piperaquini phosphatis
The invention discloses a method for preparing piperaquine phosphate. 4,7-dichloroquinoline is taken as an initial raw material and is subjected to condensation reaction with anhydrous piperazidine first to obtain 7-chloro-4-(1-piperazinyl)quinoline, then the 7-chloro-4-(1-piperazinyl)quinoline is subjected to condensation reaction with 1, 3-bromochloropropane to obtain piperaquine, and finally the piperaquine is salified with phosphorous acid to obtain the piperaquine phosphate. The method has a simple process, is easy to purify intermediate products and a final product, has high product yield and good quality, avoids the use of toxic reagents, has small pollution to the environment and low production cost, and is suitable for industrialized production.
Owner:CHONGQING KANGLE PHARMA
Medicine for treating pneumoconiosis
InactiveCN102058699AImprove subjective symptomsFunction increaseAmine active ingredientsRespiratory disorderMedicinal herbsPhosphate
The invention relates to a medicine for treating pneumoconiosis, which has simple preparation method and low cost. Zinc gluconate, piperaquine phosphate, tilorone dihydrochloride and traditional Chinese medicinal herbs fourstamen prismatomeris root and licorice are used as main ingredients of the medicine. The medicine is orally administered in the form of tablets, so as to be convenient in carrying and administration for patients. Meanwhile, the medicine has good curative effect on pneumoconiosis, and is suitable for pneumoconiosis patients at various ages and stages.
Owner:许海涛
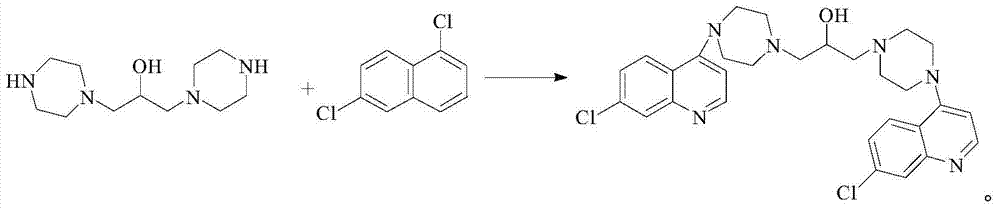
Method for synthesizing piperaquine intermediate in continuous flow microreactor
The invention discloses a method for synthesizing a piperaquine intermediate by means of a microchannel reactor, and belongs to the technical field of synthesis of antimalarial medicines. The method comprises the steps of dissolving piperaquine in an appropriate amount of water as a material I, dissolving 4,7-dichloroquinoline in an organic solvent as a material, and transporting the material I toa preheating module of the microchannel reactor for preheating; and transporting the preheated material I to a reaction module set of the microchannel reactor, meanwhile, directly transporting the material into the reaction module set of the microchannel reactor, making the material I and the material subjected to condensation reaction, collecting reaction liquid which flows out of an outlet of the microchannel reactor, and obtaining 7-chloro-4-piperazinoquinoline after conducting post-treatment on the reaction liquid. By means of the synthesis method, reaction time can be effectively shortened, so that acid non-soluble substance impurities are greatly reduced.
Owner:CHONGQING KANGLE PHARMA
Dihydroartemisinin piperaquine dry suspension agent and preparation process thereof
InactiveCN105380948AEasy to prepareEasy to operateOrganic active ingredientsInorganic non-active ingredientsPhosphateDihydroartemisinin
The invention belongs to the field of medical preparations and in particular provides a dihydroartemisinin piperaquine dry suspension agent. The dihydroartemisinin piperaquine dry suspension agent is prepared from dihydroartemisinin, piperaquine phosphate, sodium carbonate and pharmaceutically acceptable auxiliary materials, wherein the weight ratio of the dihydroartemisinin to the piperaquine phosphate to the sodium carbonate is (10 to 30) : (140 to 180) : (10 to 46). The technology can be used for remarkably improving the bitter taste of the piperaquine phosphate and improving the compliance of clinical medication for patients, and is more suitable for medication for children patients.
Owner:贝克诺顿(浙江)制药有限公司
Dihydroartemisinin piperaquine tablet and preparation method thereof
InactiveCN113057946AImprove stabilityImprove bioavailabilityOrganic active ingredientsPill deliveryActive agentSurface-active agents
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a method for preparing a solid dispersion by mixing dihydroartemisinin and piperaquine phosphate through combination of a solid dispersion method and a surfactant addition method and developing the solid dispersion into tablets, and the solid dispersion tablets prepared by the method disintegrate rapidly in water or gastric juice under the combined action of a disintegrating agent, the hydrophilic matrix is dissolved, the drug molecules are dissolved, the supersaturated state is formed in the aqueous medium without precipitation, and the two drugs are rapidly dissolved in the gastric emptying time so as to improve the bioavailability of the drugs.
Owner:南京致中生物科技有限公司
Control method of piperaquine phosphate impurity
ActiveCN104402815ASimple processWide variety of sourcesOrganic chemistryComponent separationActivated carbonReflux
The invention relates to the field of piperaquine phosphate impurity control, and specifically relates to a control method of piperaquine phosphate impurity. The control method of piperaquine phosphate impurity comprises the following steps: 1), adding a non-polar solvent into a piperaquine phosphate crude product, stirring and filtering; 2), adding a polar solvent, conducting reflux, stirring and filtering; 3), adding a water / polar mixed solvent, stirring and dissolving at 90-110 DEG, adding activated carbon for decoloring and filtering to obtain a filtrate, cooling, crystallizing, filtering, washing and drying. The steps 1) 2) and 3) are not in a particular order. The control method of piperaquine phosphate impurity provided by the invention has the advantages of simple process, and extensive equipment and reagent sources, thus indirectly reducing cost for drug impurity removal, and can be widely applied to preparation of piperaquine phosphate.
Owner:GUILIN PHARMA
Piperaquine microcapsules and compositions containing them
InactiveUS20140322296A1Preventing and minimizing degradationBad tastePowder deliveryOrganic active ingredientsMedicineQuinoline
The present invention provides a microcapsule pharmaceutical composition of at least a bisquinoline drug. said microcapsule comprises a drug core of a pharmaceutically effective amount of a bisquinoline drug and a polymeric coating over the core. This microcapsule pharmaceutical composition has desirable pharmaceutical properties, including taste masking effect and a high stability.
Owner:ADARE PHARM SRL
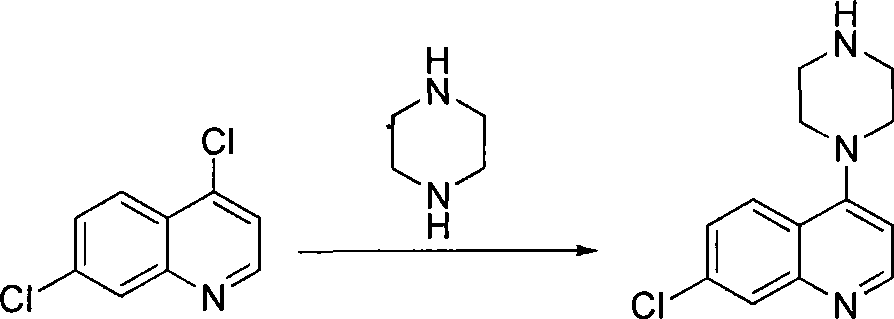
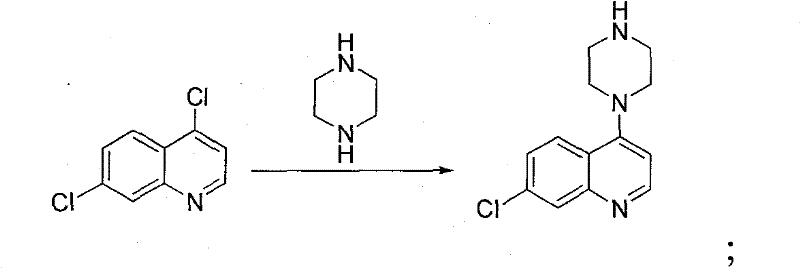
Novel Process for the Synthesis of 7-Chloro-4-(piperazin-1-yl)-quinoline
InactiveUS20140200346A1Large scaleHigh cost-effectiveAntibacterial agentsOrganic active ingredientsQuinolineImpurity
The present invention provides a new process of synthesis of a polymorph of 7-chloro-4-(piperazin-1-yl)-quinoline of Formula I. Said quinoline compound is substantially pure of any impurities. The present invention further provides the use of the above-mentioned polymorph of 7-chloro-4-(piperazin-1-yl)-quinoline in the synthesis of piperaquine or one of its pharmaceutically acceptable salts.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Piperaquine phosphate oral liquid and preparation method thereof
InactiveCN107929240AReduce manufacturing costQuality improvementOrganic active ingredientsDispersion deliveryOlder peoplePhosphate
The invention provides piperaquine phosphate oral liquid and a preparation method thereof. The piperaquine phosphate oral liquid is prepared from the following components in parts by weight: 1 part ofpiperaquine phosphate, 5-15 parts of water, 1-2 parts of ethanol and a pH regulator; the pH value of the oral liquid is 2.5-4. The preparation method comprises the following steps: mixing piperaquinephosphate, water and ethanol, and regulating the pH value to 2.5-4 by use of the pH regulator to obtain a piperaquine phosphate solution; adding the rest raw materials into the solution to obtain theproduct. The oral liquid provided by the invention has the advantages of relatively fast absorption, stable quality and the like, and is suitable for the old people, children and patients suffering dysphagia, and is easy to take; moreover, with strong efficacy, the oral liquid is a good substitute of piperaquine phosphate tablets.
Owner:GUILIN PHARMA
Application of piperaquine nitrogen oxide in preparation of medicines for resisting malaria
InactiveCN105982898AScientific and rigorous experimental designEnhanced inhibitory effectOrganic active ingredientsAntiparasitic agentsMedicineIn vitro test
The invention discloses application of piperaquine mono-nitrogen oxide and / or piperaquine dinitrogen oxide or pharmaceutically acceptable salt in preparation of medicines for resisting malaria. According to the unexpected discovery, piperaquine mono-nitrogen oxide and piperaquine dinitrogen oxide have a remarkable inhibition effect on growth of plasmodium and development of agamont. In-vitro tests prove that the piperaquine mono-nitrogen oxide and piperaquine dinitrogen oxide show the activity of inhibiting development of plasmodium agamont at a relatively low concentration (7.8nM); and in-vivo tests prove that oral piperaquine mono-nitrogen oxide and piperaquine dinitrogen oxide have a remarkable effect on inhibiting plasmodium falciparum.
Owner:SHANDONG UNIV
Process for the synthesis of 7-chloro-4-(piperazin-1-yl)-quinoline
InactiveUS9206133B2Easily feasibleHigh cost-effectiveAntibacterial agentsOrganic active ingredientsQuinolinePiperazine
The present invention provides a new process of synthesis of a polymorph of 7-chloro-4-(piperazin-1-yl)-quinoline of Formula I. Said quinoline compound is substantially pure of any impurities. The present invention further provides the use of the above-mentioned polymorph of 7-chloro-4-(piperazin-1-yl)-quinoline in the synthesis of piperaquine or one of its pharmaceutically acceptable salts.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
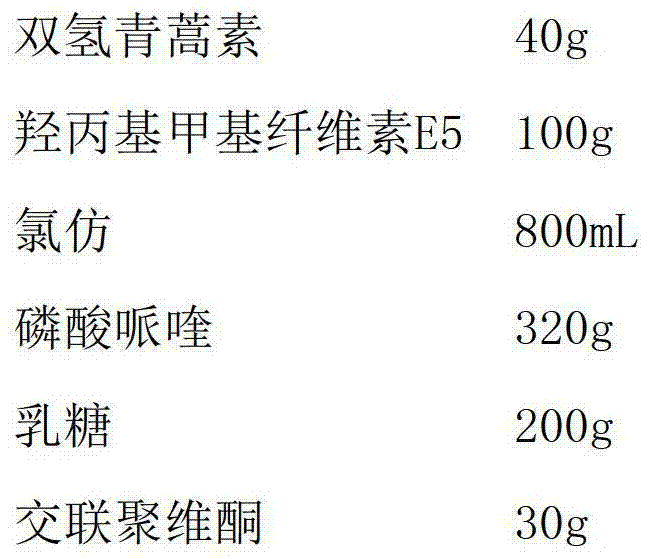
A kind of dihydroartemisinin piperaquine phosphate tablet and preparation method thereof
ActiveCN103263418BPromote dissolutionReduce onset timeOrganic active ingredientsPill deliveryPhosphateMethyl cellulose
The invention discloses dihydroartemisinin piperaquine phosphate tablets and a preparation method thereof. The tablets are prepared from dihydroartemisinin, piperaquine phosphate, hydroxypropyl methyl cellulose E5, hydrophilic accessory and lubricating agent, wherein the weight ratio of the dihydroartemisinin to the hydroxypropyl methyl cellulose E5 is 1:(1-5). Solid dispersion of the dihydroartemisinin and pulverized piperaquine phosphate can be obtained by adopting one-step operation, so that the production efficiency is improved; and the tablets are dissolved quickly.
Owner:贝克诺顿(浙江)制药有限公司
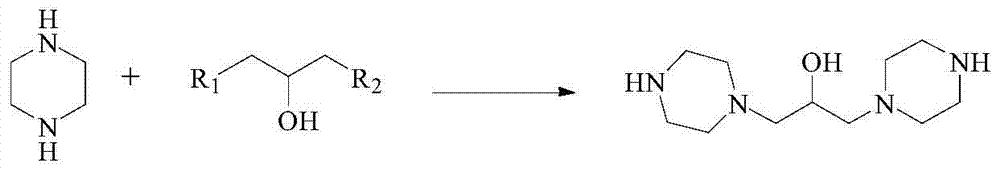
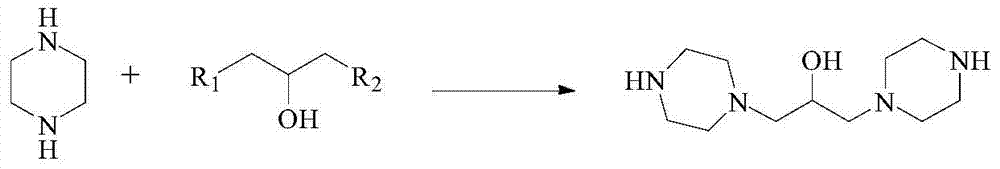
Preparation method of hydroxypiperaquine and phosphate thereof
The invention provides a preparation method of hydroxypiperaquine and phosphate thereof. The preparation method of hydroxypiperaquine comprises the following steps: by using piperazine, 1,3-dihalopropanol and 4,7-dichloroquinoline as raw materials, reacting the piperazine and 1,3-dihalopropanol by using an acid-binding agent as a catalyst to obtain 1,3-dipiperazinylpropanol, carrying out condensation reaction on the 1,3-dipiperazinylpropanol and 4,7-dichloroquinoline under the catalytic action of an alkali to obtain the hydroxypiperaquine. The obtained hydroxypiperaquine reacts with phosphoric acid to obtain the hydroxypiperaquine phosphate. The method has the advantages of mild reaction conditions, simple preparation technique and low cost, and is suitable for industrial production. The total reaction yield of the hydroxypiperaquine is up to 90-91.5%, and the total reaction yield of the hydroxypiperaquine phosphate is up to 82.7-87.5%.
Owner:上海博速医药科技有限公司
Preparation method of hydroxypiperaquine and phosphate thereof
ActiveCN105111142AEasy to prepareEasy to operateOrganic chemistryPhosphoric acidHydroxypiperaquine phosphate
The invention provides a preparation method of hydroxypiperaquine and phosphate thereof. The preparation method of hydroxypiperaquine comprises the following steps: by using piperazine, 1,3-dihalopropanol and 4,7-dichloroquinoline as raw materials, reacting the piperazine and 1,3-dihalopropanol by using an acid-binding agent as a catalyst to obtain 1,3-dipiperazinylpropanol, carrying out condensation reaction on the 1,3-dipiperazinylpropanol and 4,7-dichloroquinoline under the catalytic action of an alkali to obtain the hydroxypiperaquine. The obtained hydroxypiperaquine reacts with phosphoric acid to obtain the hydroxypiperaquine phosphate. The method has the advantages of mild reaction conditions, simple preparation technique and low cost, and is suitable for industrial production. The total reaction yield of the hydroxypiperaquine is up to 90-91.5%, and the total reaction yield of the hydroxypiperaquine phosphate is up to 82.7-87.5%.
Owner:上海博速医药科技有限公司
Application of piperaquine phosphate in the preparation of drugs for for treating lipid metabolism disorder and hyperlipidemia
ActiveCN109172578AReduce contentGood treatment effectOrganic active ingredientsMetabolism disorderSerum igeTreatment effect
The invention relates to the pharmaceutical field, in particular to the application of piperaquine phosphate in the preparation of drugs for treating lipid metabolism disorder and hyperlipidemia. Piperaquine phosphate can significantly reduce serum TC and TG levels, significantly reduce blood lipid, and can significantly reduce body weight, and no obvious toxic and side effects, which can be usedto treat hyperlipidemia, and can be used in the preparation of hyperlipidemia drugs.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Stable dosage forms of arterolane and piperaquine
The field of the invention relates to stable oral dosage forms comprising, (a) cis-adamantane-2-spiro-3'-8'-[[[(2'-amino-2'-methylpropyl)amino]carbonyl]-methyl]-l',2',4'-trioxaspiro[4.5]decane hydrogen maleate(Active compound I); (b) piperaquine; and (c) one or more pharmaceutically acceptable excipients; and processes for their preparation, especially wherein the dosage form is prepared by a dry process.
Owner:RANBAXY LAB LTD
Compositions and methods of ameliorating pharmaceutical aversiveness with salts
PendingUS20210007977A1Improve complianceSuppress aversivenessOrganic active ingredientsDispersion deliveryBitter tastesPharmaceutical Substances
Provided herein are compositions and methods of ameliorating pharmaceutical aversiveness via salt. In one aspect, a composition is provided comprising Praziquantel, and an effective amount of Na Gluconate which suppresses bitter taste of orally administrated Praziquantel. Additionally, a composition is provided comprising Piperaquine and an effective amount of KOH which suppresses aversiveness of orally administrated Piperaquine.
Owner:MONELL CHEM SENSES CENT
Polycrystal substance of piperaquine phosphate and preparation method thereof
ActiveCN102558048BLow toxicityProcess parameters are easy to controlOrganic chemistryCrystallographyBiotechnology
The invention discloses a novel polycrystal substance of piperaquine phosphate. Compared with amorphous piperaquine phosphate, the polycrystal substance of the piperaquine phosphate, which is disclosed by the invention, has better stability and better facilitates the production, the storage and the circulation of raw medicine and preparations thereof, wherein a crystal form A of the piperaquine phosphate has the best stability, the best dissolution rate and the best dissolubility. The invention further discloses a preparation method of the polycrystal substance of the piperaquine phosphate. The method has the advantages of low toxicity, people friendliness, environment friendliness, easily-controlled technological parameters, and the like, is simple to operate and is suitable for mass production.
Owner:珠海润都制药股份有限公司
The control method of piperaquine phosphate impurity
ActiveCN104402815BSimple processWide variety of sourcesOrganic chemistryComponent separationRefluxActivated carbon
The invention relates to the field of piperaquine phosphate impurity control, and specifically relates to a control method of piperaquine phosphate impurity. The control method of piperaquine phosphate impurity comprises the following steps: 1), adding a non-polar solvent into a piperaquine phosphate crude product, stirring and filtering; 2), adding a polar solvent, conducting reflux, stirring and filtering; 3), adding a water / polar mixed solvent, stirring and dissolving at 90-110 DEG, adding activated carbon for decoloring and filtering to obtain a filtrate, cooling, crystallizing, filtering, washing and drying. The steps 1) 2) and 3) are not in a particular order. The control method of piperaquine phosphate impurity provided by the invention has the advantages of simple process, and extensive equipment and reagent sources, thus indirectly reducing cost for drug impurity removal, and can be widely applied to preparation of piperaquine phosphate.
Owner:GUILIN PHARMA
High-yield piperaquine preparation method
InactiveCN109438346AEasy to separate and purifyLess impuritiesOrganic chemistryAmmonium hydroxideImpurity
The invention discloses a high-yield piperaquine preparation method and belongs to the technical field of anti-malarial medicine preparation. The preparation method comprises the following steps: firstly, utilizing piperaquine hydrochloride as a starting material, adding hot water, stirring to completely dissolve the starting material while heating the starting material and filtering to obtain a piperaquine hydrochloride solution; then, adding ammonium hydroxide into the obtained piperaquine hydrochloride solution to perform neutral reaction and filtering to obtain piperaquine precipitate; finally, adding purified water into the obtained piperaquine precipitate and repeatedly washing, dewatering and drying to obtain piperaquine. According to the preparation method disclosed by the invention, the piperaquine hydrochloride is utilized as the starting material to directly perform neutral reaction with ammonium hydroxide to obtain piperaquine through one-step transformation. As the piperaquine is insoluble in water, the piperaquine is easy to separate and purify and has the advantages of small impurities, high purity and high product yield. In addition, the technical scheme of the preparation method disclosed by the invention further avoids use of toxic reagents and has the advantages of small work procedures, low production cost and suitability for industrial production.
Owner:ARTEPHARM CO LTD CHINA
Compound artemisinin piperaquine pellet and preparation method thereof
ActiveCN102485226BSimple preparation processShape ruleOrganic active ingredientsPharmaceutical non-active ingredientsPhosphateLactose
Owner:KPC PHARM INC
Medicine for treating pneumoconiosis
InactiveCN102058699BImprove subjective symptomsFunction increaseAmine active ingredientsRespiratory disorderMedicinal herbsAdditive ingredient
Owner:许海涛
A kind of method that piperazine is applied mechanically to produce piperaquine phosphate intermediate quinoline piperazine hydrochloride
Owner:珠海润都制药股份有限公司
Piperaquine microcapsules and compositions containing them
ActiveUS20160045447A1Improve the effect of chemical reactionsEasily undergoes degradation reactionOrganic active ingredientsPill deliveryMedicinePolymer
The present invention provides a microcapsule pharmaceutical composition of at least a bisquinoline drug. said microcapsule comprises a drug core of a pharmaceutically effective amount of a bisquinoline drug and a polymeric coating over the core. This microcapsule pharmaceutical composition has desirable pharmaceutical properties, including taste masking effect and a high stability.
Owner:ADARE PHARM SRL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com