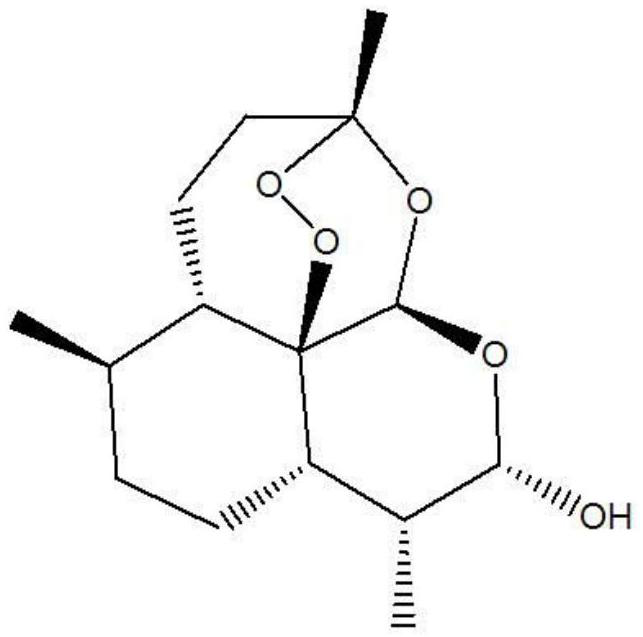
Dihydroartemisinin piperaquine tablet and preparation method thereof
A technology of dihydroartemisinin and piperaquine tablets, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, and medical preparations containing active ingredients. cumbersome process and other issues, to achieve the effect of improving stability and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] A kind of preparation method of dihydroartemisinin-piperaquine tablet in the present embodiment, comprises the steps:
[0052] 1) Solid dispersion
[0053] Form a solution of the solvent hydrophilic matrix, surfactant and co-solvent at 60°C, keep warm at 40°C, add the prescribed amount of hydrogen artemisinin and piperaquine phosphate in sequence, stir until dissolved, and cool the obtained hot mixture , and dried the obtained solid dispersion at a temperature of 35 to 40°C.
[0054] 2) Preparation of dihydroartemisinin-piperaquine tablets
[0055] Put the solid dispersion and colloidal silicon dioxide of the prescribed amount into a low-shear V-shaped mixer and mix for 10 minutes, then put the prescribed amount of cellulose lactose and sodium carboxymethyl starch into the low-shear V-shaped mixer for Mix, mix 30min, add the magnesium stearate of recipe quantity, then mix in the low-shear V-type mixer, mix 5min, the mixed powder obtained is poured into the mold of sha...
Embodiment 2
[0059] A kind of preparation method of dihydroartemisinin-piperaquine tablet in the present embodiment, comprises the steps:
[0060] 1) Solid dispersion
[0061] Form a solution of the solvent hydrophilic matrix, surfactant and co-solvent at 60°C, keep warm at 40°C, add the prescribed amount of hydrogen artemisinin and piperaquine phosphate in sequence, stir until dissolved, and cool the obtained hot mixture , and dried the obtained solid dispersion at a temperature of 35 to 40°C.
[0062] 2) Preparation of dihydroartemisinin-piperaquine tablets
[0063] Put the solid dispersion and colloidal silicon dioxide of the prescribed amount into a low-shear V-shaped mixer and mix for 10 minutes, then put the prescribed amount of cellulose lactose and sodium carboxymethyl starch into the low-shear V-shaped mixer for Mix, mix 30min, add the magnesium stearate of recipe quantity, then mix in the low-shear V-type mixer, mix 5min, the mixed powder obtained is poured into the mold of sha...
Embodiment 3
[0067] A kind of preparation method of dihydroartemisinin-piperaquine tablet in the present embodiment, comprises the steps:
[0068] 1) Solid dispersion
[0069] Form a solution of the solvent hydrophilic matrix, surfactant and co-solvent at 60°C, keep warm at 40°C, add the prescribed amount of hydrogen artemisinin and piperaquine phosphate in sequence, stir until dissolved, and cool the obtained hot mixture , and dried the obtained solid dispersion at a temperature of 35 to 40°C.
[0070] 2) Preparation of dihydroartemisinin-piperaquine tablets
[0071] Put the solid dispersion and colloidal silicon dioxide of the prescribed amount into a low-shear V-shaped mixer and mix for 10 minutes, then put the prescribed amount of cellulose lactose and sodium carboxymethyl starch into the low-shear V-shaped mixer for Mix, mix 30min, add the magnesium stearate of recipe quantity, then mix in the low-shear V-type mixer, mix 5min, the mixed powder obtained is poured into the mold of sha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com