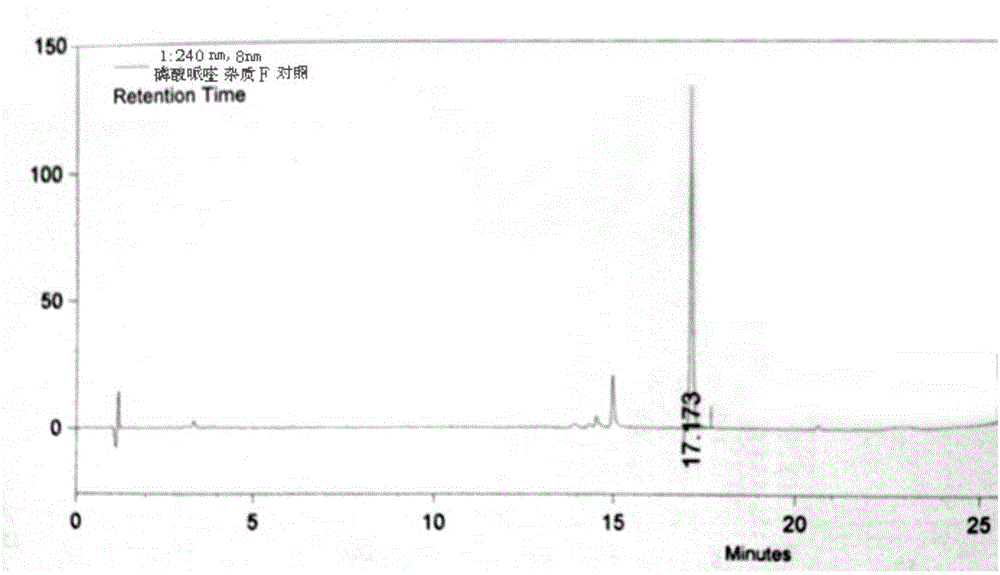
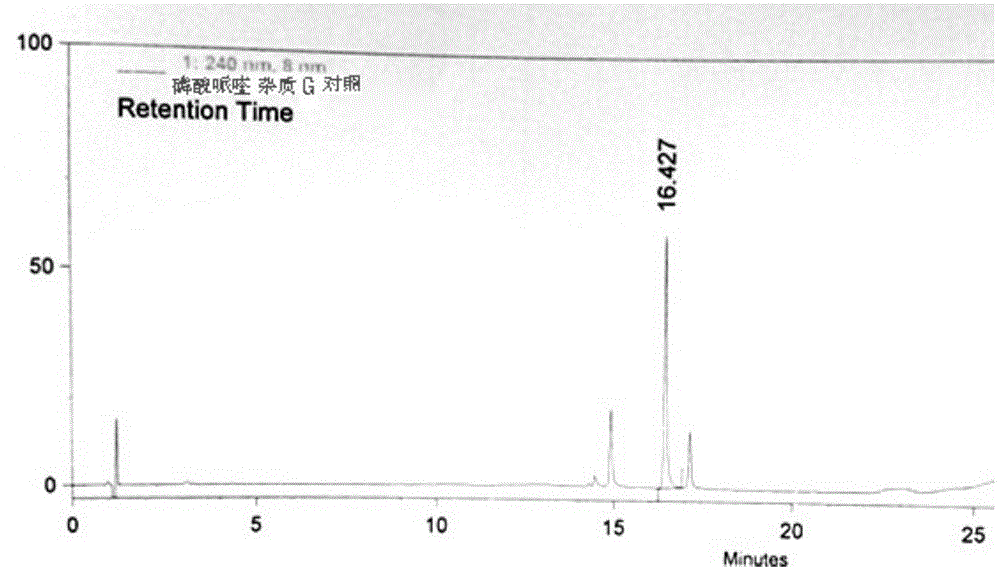
Control method of piperaquine phosphate impurity
A piperaquine phosphate and control method technology, which is applied in the field of piperaquine phosphate impurity control, can solve the problems that impurity control methods are difficult to remove, and achieve the effects of wide sources of equipment and medicaments, simple process, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The method for controlling the impurities of piperaquine phosphate provided in this example includes: adding crude piperaquine phosphate into a non-polar solvent, stirring and then filtering to obtain a primary purified solid. The primary purified solid is added into a polar solvent, refluxed and stirred, and filtered to obtain a filtered solid, and the filtered solid is dried to obtain a secondary purified solid. The secondary purified solid was added into a polar mixed solvent, stirred and dissolved at 100° C., and then activated carbon was added for decolorization and then filtered to obtain a filtrate. The filtrate is cooled and crystallized, and then filtered, washed and dried to obtain purified piperaquine phosphate.
Embodiment 2
[0043] The method for controlling the impurities of piperaquine phosphate provided in this example includes: adding the crude piperaquine phosphate to ethyl acetate whose weight is 9 times its weight, stirring at 50° C. for 1.5 hours and then filtering to obtain a primary purified solid. The primary purified solid was added into ethanol, refluxed and stirred for 1.5 hours at a temperature of 35°C, and then filtered to obtain a filtered solid, which was dried to obtain a secondary purified solid. The secondary purified solid was added to a mixed solution of water and ethanol with a volume ratio of 100:1, stirred and dissolved at 90° C., and activated carbon was added to incubate for 28 minutes and then filtered to obtain a filtrate. The filtrate was cooled and crystallized at room temperature for 7 hours, followed by filtration, washing with purified water and drying to obtain purified piperaquine phosphate.
Embodiment 3
[0045] The method for controlling the impurities of piperaquine phosphate provided in this example comprises: adding the crude piperaquine phosphate into methylene chloride 10 times its weight, stirring at 55° C. for 2 hours and then filtering to obtain a primary purified solid. The primary purified solid was added into acetone, refluxed and stirred for 2 hours at a temperature of 70°C, and then filtered to obtain a filtered solid, which was dried to obtain a secondary purified solid. The secondary purified solid was added to a mixed solution of water and ethanol with a volume ratio of 500:1, stirred and dissolved at 100° C., and then activated carbon was added to incubate for 30 minutes and then filtered to obtain a filtrate. The filtrate was cooled and crystallized at room temperature for 8 hours, followed by filtration, washing with purified water and drying to obtain purified piperaquine phosphate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com