Preparation methods of lenvatinib mesylate drug impurities
A technology of lenvatinib mesylate and a compound, which is applied in the field of drug synthesis and can solve problems such as no report on process impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Synthesis of impurity A
[0035] Impurity A, 4,4'-(((Carbonylbis(ureadiyl))bis(3-chloro-4,1-phenyl))bis(oxy))bis(7-methoxyquinoline-6 -Formamide) synthesis
[0036] Add 20ml of N-methylpyrrolidone to a 100ml three-necked flask, add 1.00g LVTN-1, 0.92g pyridine, nitrogen protection, and cool to 0~10°C under stirring. Start adding 1.46g phenyl chloroformate dropwise. , Continue to stir for 20-30 minutes, increase the temperature to 60°C, and stir overnight; take a sample of TLC to detect (methanol:dichloromethane=1:10) that the raw material disappears. Add 20ml of water, a large amount of solids will precipitate out, continue stirring for 30 minutes. Filter, drain, and blast dry at 40°C for 30 minutes to obtain a crude solid product. The crude product is purified by column chromatography. The elution ratio is: methanol:dichloromethane=1:50. Collect the eluate in a total of 160mL, and control the temperature. 30~40℃, vacuum degree: -0.08MPa, vacuum distillation, e...
Embodiment 2
[0044] Example 2: Synthesis of impurity B
[0045] Impurity B, the synthesis of 4-(3-chloro-4-(3,3-dimethylureido)phenoxy)-7-methoxyquinoline-6-carboxylic acid amide.
[0046] To a 50ml three-necked flask were added 463.87mg of levatinib mesylate intermediate LVTN-2 and 9mL of N-methylpyrrolidone. The reaction temperature was controlled at 0-10°C, 99.18 mg dimethylamine was added to the reaction system, and stirring was continued for 30 minutes, monitored by TLC (dichloromethane:methanol=10:1), and the reaction was completed. 18 mL of 80% acetone / water (V / V) mixed solvent was added to the reaction system, and a large amount of solids were precipitated. After stirring for 30 minutes, it was filtered and air-dried at 60° C. to obtain crude impurity B. The crude product was purified by column chromatography. The developing solvent and the ratio were: methanol:dichloromethane=1:20 (V / V), and a total of 150 mL of eluent was collected. The temperature was controlled at 30-40°C, and the ...
Embodiment 3
[0047] Example 3: Synthesis of impurity C
[0048] Impurity C, the synthesis of 4-ethoxy-7-methoxyquinoline-6-carboxamide
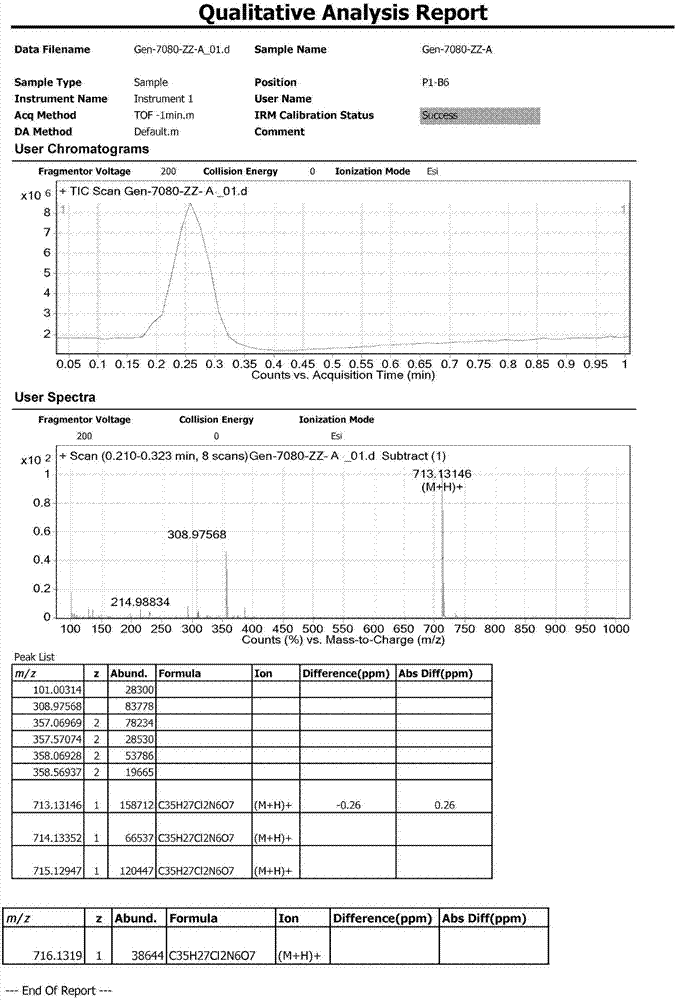
[0049] Add 2.00 g of lenvatinib mesylate and 50 mL of ethanol to a 100 ml three-necked flask. Stir under reflux for 72 hours, monitored by TLC (methanol:dichloromethane=1:10, new spots are generated). The solvent was evaporated under reduced pressure, and the crude product obtained was purified by column chromatography. The elution ratio was: methanol:dichloromethane=1:20, and a total of 60 mL of eluate was collected. The temperature was controlled at 30-40°C, and the vacuum degree: -0.08MPa Under reduced pressure distillation, the solvent was distilled off until no distillate was evaporated to obtain 122 mg of pale pink solid, yield: 6.10%, namely impurity C: 4-ethoxy-7-methoxyquinoline-6-carboxamide.
[0050]
[0051] Impurity C
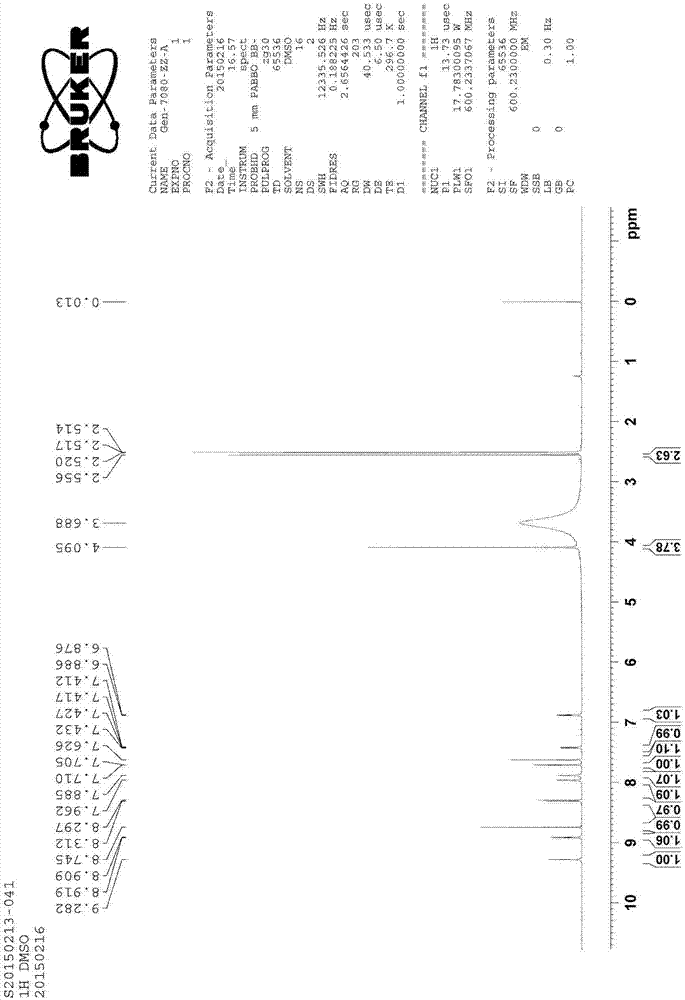
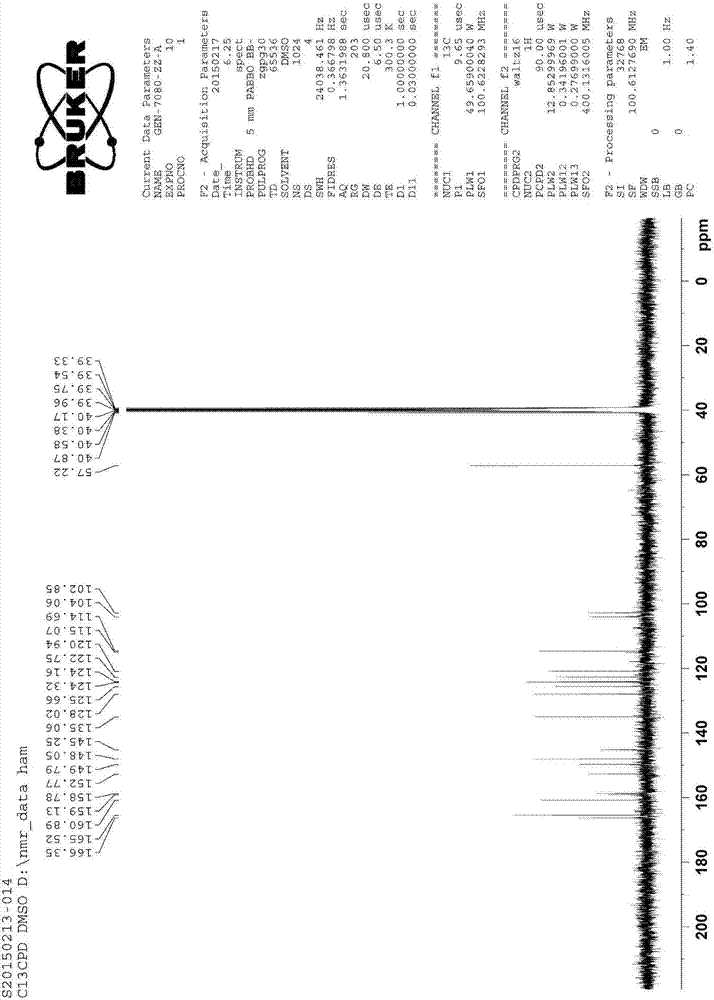
[0052] The structure confirmation of impurity C is shown in the following table:
[0053] Table 2 Impurity C 1 H-NMR and 13 C-NMR t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com