Preparation method of prostaglandin medicine impurity
A technology of prostaglandin and precursor ketone, applied in the field of drug synthesis, can solve the problems of high price of chiral column, high economic cost and high equipment requirements, and achieve the advantages of easy availability and low price of raw materials, simple processing and simple reaction operation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
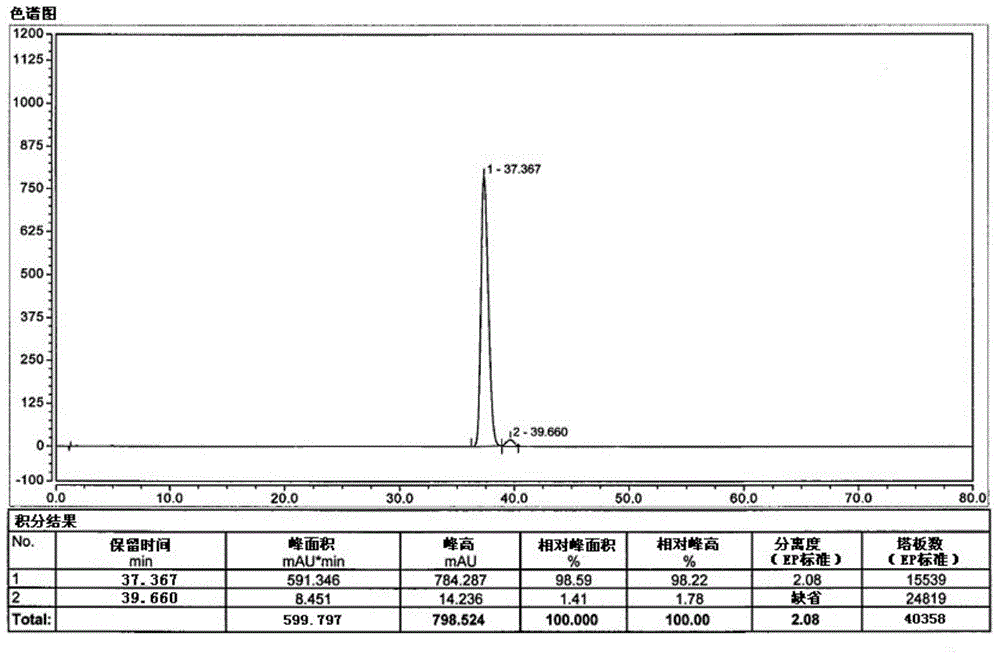
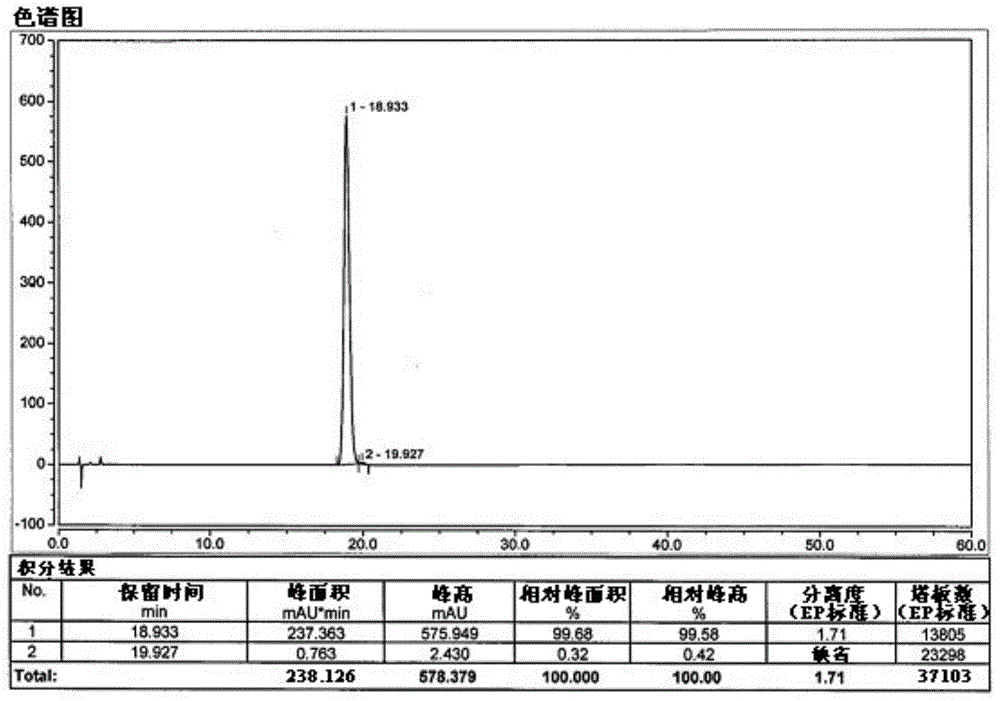
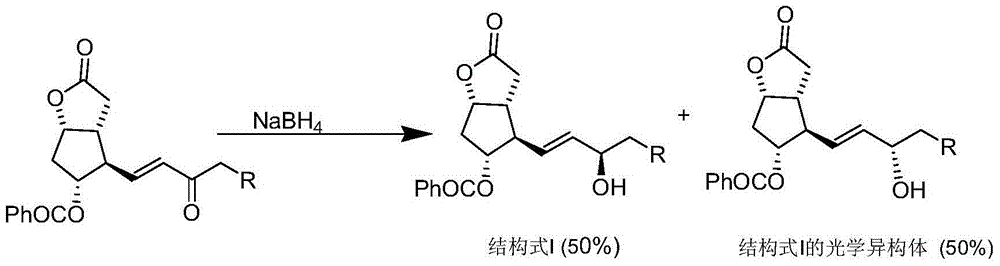
[0028] Under nitrogen protection, 0.095 g (2.5 mmol) of sodium borohydride was added to a 250 mL three-necked flask, and 2.040 g (-)-α-pinene (95% ee, 15 mmol) was added dropwise at -10°C. Below 10°C, add 1.0M BCl dropwise 3 2.5mL n-hexane solution (containing BCl 3 2.5mmol), react at 10°C for 1h, then raise the temperature to 30-40°C for 1h. Cool down to -25~-15°C, add A tetrahydrofuran solution (A: 1.0g2.5mmol, dissolved in 10mL tetrahydrofuran) dropwise, and stir for 10h. After the reaction was detected by TLC, the reaction solution was concentrated under reduced pressure, 10 mL of water and 10 mL of ethyl acetate were added to the concentrated solution, and the layers were separated. The aqueous layer was extracted with ethyl acetate (10 mL×2). Wash twice, separate the layers, dry the ethyl acetate layer by adding anhydrous sodium sulfate, filter out the sodium sulfate, concentrate the filtrate under reduced pressure, and purify by column chromatography to obtain 0.81 g ...
Embodiment 2
[0030] Under nitrogen protection, 0.190 g (5 mmol) of sodium borohydride was added to a 250 mL three-necked flask, and 2.040 g (-)-α-pinene (95% ee, 15 mmol) was added dropwise at -10°C. Below 10°C, add 1.0M BCl dropwise 3 5.0mL n-hexane solution (containing BCl 3 5mmol), react at 10°C for 1h, then raise the temperature to 30-40°C for 1h. Cool down to -25~-15°C, add A tetrahydrofuran solution (A: 1.0g2.5mmol, dissolved in 10mL tetrahydrofuran) dropwise, and stir for 10h. After the reaction was detected by TLC, the reaction solution was concentrated under reduced pressure, 10 mL of water and 10 mL of ethyl acetate were added to the concentrated solution, and the layers were separated. The aqueous layer was extracted with ethyl acetate (10 mL×2). Wash twice, separate the layers, dry the ethyl acetate layer by adding anhydrous sodium sulfate, filter out the sodium sulfate, concentrate the filtrate under reduced pressure, and purify by column chromatography to obtain 0.86 g of o...
Embodiment 3
[0032] Under nitrogen protection, 0.283 g (7.5 mmol) of sodium borohydride was added to a 250 mL three-necked flask, and 2.040 g (-)-α-pinene (95% ee, 15 mmol) was added dropwise at -10°C. Below 10°C, add 1.0M BCl dropwise 3 7.5mL n-hexane solution (containing BCl 3 7.5mmol), react at 10°C for 1h, then raise the temperature to 30-40°C for 1h. Cool down to -25~-15°C, add A tetrahydrofuran solution (A: 1.0g2.5mmol, dissolved in 10mL tetrahydrofuran) dropwise, and stir for 10h. After the reaction was detected by TLC, the reaction solution was concentrated under reduced pressure, 10 mL of water and 10 mL of ethyl acetate were added to the concentrated solution, and the layers were separated. The aqueous layer was extracted with ethyl acetate (10 mL×2). Wash twice, separate the layers, dry the ethyl acetate layer by adding anhydrous sodium sulfate, filter out the sodium sulfate, concentrate the filtrate under reduced pressure, and purify by column chromatography to obtain 0.83 g ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com