Method for preparing azide-substituted quinoline-2, 4-diketone through free radical tandem carbon cyclization reaction without metal catalysis
A metal-free catalysis, free-radical technology, used in organic chemistry, chemicals for biological control, fungicides, etc., can solve the problems of increased probability of side reactions, high production costs, and environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
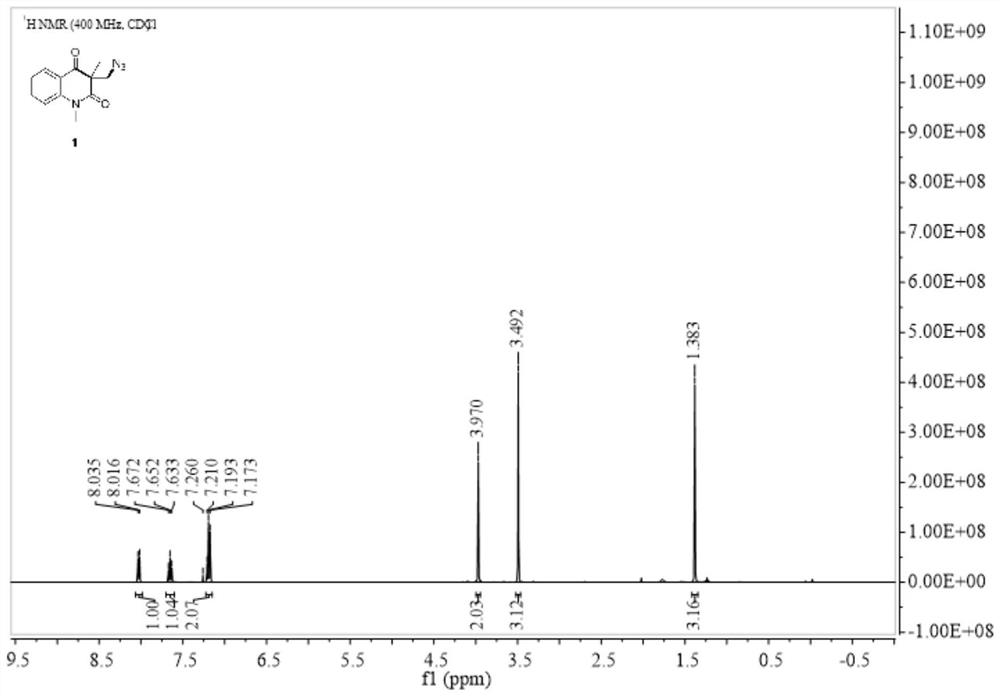
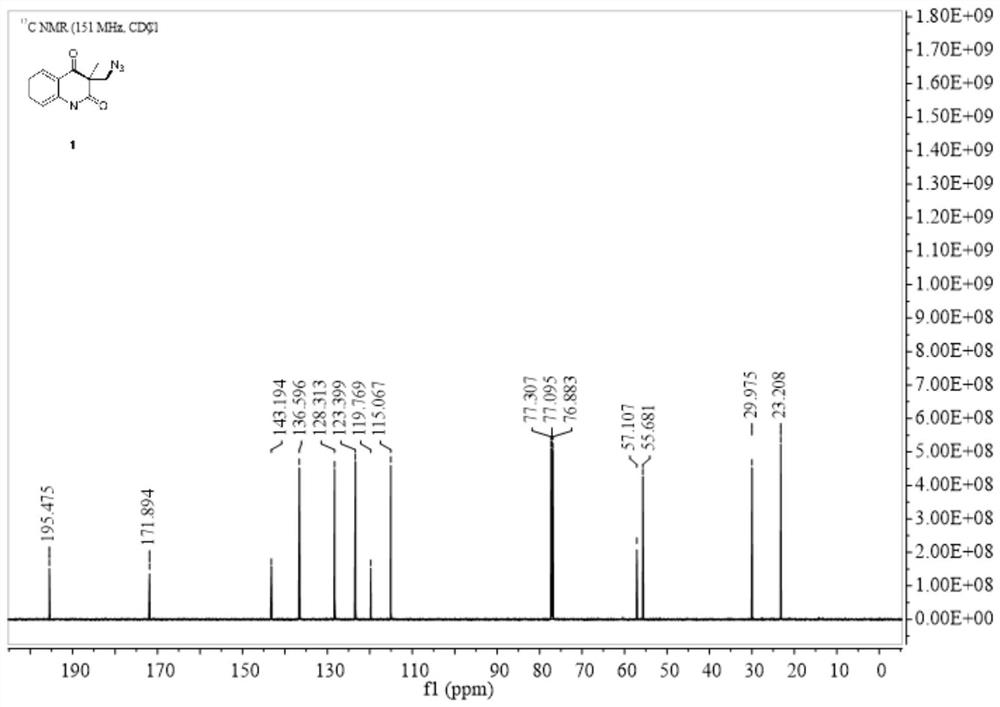
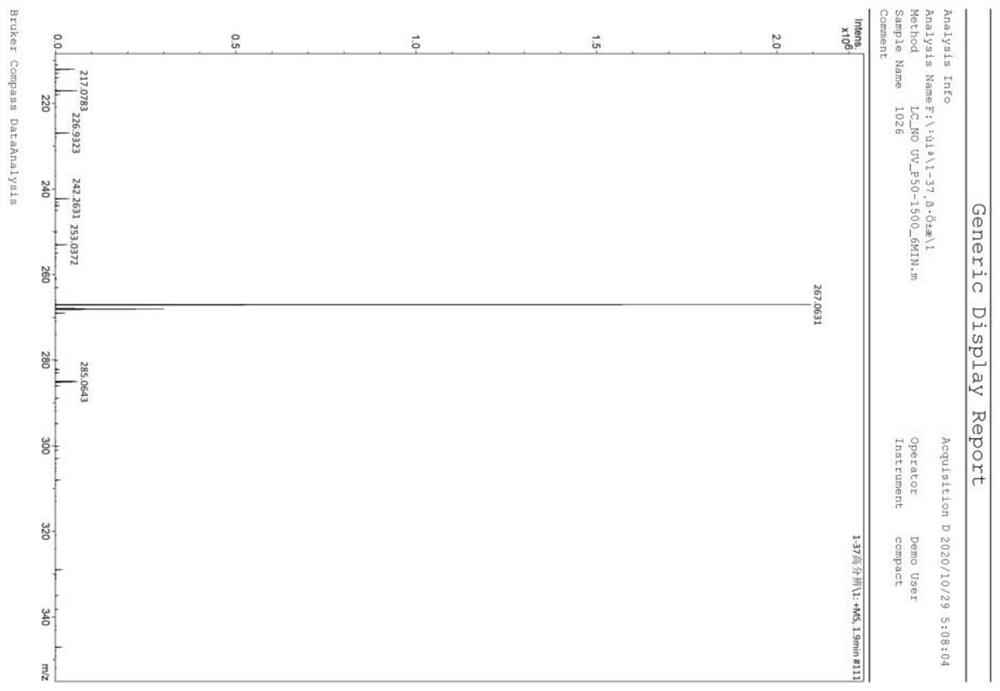
[0034] Embodiment 1: In this embodiment, the method for preparing azide-substituted quinoline-2,4-dione by metal-free catalyzed series carbocyclization reaction is as follows:
[0035] At room temperature, add N-(2-cyanophenyl)-N-methacrylamide (1a) 60mg, diphenyldiselenide (PhSe) 2 46.8 mg, 145 mg of iodobenzenediethyl ester and 127 μL of azidotrimethylsilane (specification 93%) were finally reacted in 2 mL of dimethyl sulfoxide (DMSO), and the reaction tube was stirred at room temperature for 12 hours until Monitored by TLC analysis until the raw material is completely consumed, after the reaction is complete, the mixture was washed with H 2 O (15 mL) quenched with CH 2 Cl 2 (3×5mL) extraction, then the organic solvent was concentrated in vacuo, and the residue was purified by flash column chromatography, using a mixture of petroleum ether and ethyl acetate as eluent, Rf=0.44, to obtain 3-(azidomethyl) - 1,3-Dimethylquinoline-2,4(1H,3H)-dione (2a). Yield: 90%, 66 mg.
...
specific Embodiment approach 2
[0040] Specific embodiment 2: In this embodiment, the method for preparing azide-substituted quinoline-2,4-dione by metal-free catalyzed series carbocyclization reaction is as follows:
[0041] At room temperature, 65 mg of N-(2-cyano-3-fluorophenyl)-N-methacrylamide (1b), 46.8 mg of diphenyldiselenide, and iodobenzenediethyl ester were sequentially added to the reaction tube 145 mg and 127 μL of azidotrimethylsilane (93% strength) were finally reacted in 2 mL of dimethyl sulfoxide (DMSO) and the reaction tube was stirred at room temperature for 12 h until complete consumption of starting material as monitored by TLC analysis , after the reaction was complete, the mixture was washed with H 2 O (15 mL) quenched with CH 2 Cl 2 (3×5mL) extraction, then the organic solvent was concentrated in vacuo, and the residue was purified by flash column chromatography, using a mixture of petroleum ether and ethyl acetate as eluent, Rf=0.29, to obtain 3-(azidomethyl )-5-fluoro-1,3-dimethy...
specific Embodiment approach 3
[0045] Specific embodiment three: In this embodiment, the method for preparing azide-substituted quinoline-2,4-dione by metal-free catalyzed series carbocyclization reaction is as follows:
[0046] At room temperature, successively add 70 mg of N-(3-chloro-2-cyanophenyl)-N-methacrylamide (1c), 46.8 mg of diphenyldiselenide, and iodobenzenediethyl 145 mg and 127 μL of azidotrimethylsilane (93% strength) were finally reacted in 2 mL of dimethyl sulfoxide (DMSO) and the reaction tube was stirred at room temperature for 12 h until complete consumption of starting material as monitored by TLC analysis , after the reaction was complete, the mixture was washed with H 2 O (15 mL) quenched with CH 2 Cl 2 (3×5mL) extraction, then the organic solvent was concentrated in vacuo, and the residue was purified by flash column chromatography, using a mixture of petroleum ether and ethyl acetate as eluent, Rf=0.31, to obtain 3-(azidomethyl) -5-Chloro-1,3-dimethylquinoline-2,4(1H,3H)-dione (c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com