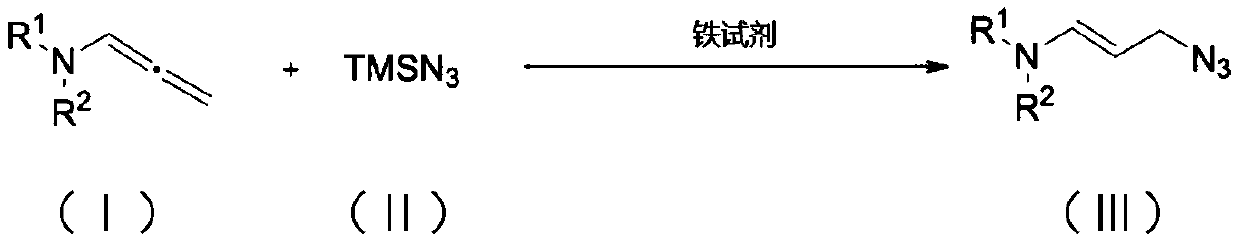
Method for synthesizing allyl azide derivative
A derivative, allyl technology, applied in the field of synthesizing allyl azide derivatives, can solve the problems of low reaction yield, explosiveness, increased cost, etc., and achieves cheap and easy-to-obtain raw materials, simple synthesis steps, and reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
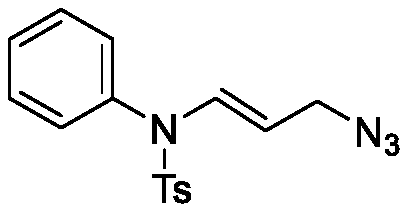
[0023] In a 25mL reaction tube, add aniline allene (28.5mg, 0.1mmol), ferrous chloride (2.5mg, 0.02mmol), azidotrimethylsilane (17.3mg, 0.15mmol), add 2mL of dichloromethane , stirred and refluxed at 40°C for 26 hours until the raw material allenamine was consumed, then the mixture was concentrated under reduced pressure and purified by chromatographic column (petroleum ether / ethyl acetate=20 / 1) to obtain a yellow solid product (30.9 mg, yield: 95%).
[0024] The structural formula of the obtained compound is as follows:
[0025]
[0026] m p 81.4-82.3℃; IR (neat) 3610, 2932, 2108, 1661, 1587, 1489, 1409, 743cm -1 ; 1 HNMR (400MHz, CDCl 3 )δ7.55(d, J=8.0Hz, 2H), 7.38–7.36(m, 3H), 7.31(d, J=12.4Hz, 1H), 7.27(d, J=2.6Hz, 2H), 6.96( d,J=6.3Hz,2H),4.40(dt,J=14.7,7.5Hz,1H),3.70(d,J=7.4Hz,2H),2.44(s,3H). 13 C NMR (100MHz, CDCl 3 )δ144.29, 135.81, 135.24, 134.30, 130.03, 129.71, 129.66, 129.34, 127.49, 103.09, 51.00, 21.64. HRMS (ESI) m / z calcd for C 16 h 17 N 4 o 2 S +...
Embodiment 2
[0028] In a 25mL reaction tube, add p-fluoroaniline (30.3mg, 0.1mmol), ferrous chloride (2.5mg, 0.02mmol), azidotrimethylsilane (17.3mg, 0.15mmol), add 2mL di Chloromethane, stirred and refluxed at 40°C for 40 hours until the raw material allenamine was consumed, then the mixture was concentrated under reduced pressure and purified by chromatography (petroleum ether / ethyl acetate=20 / 1) to obtain a white solid product (33.6mg, produced The rate is 97%). The structural formula of the obtained compound is as follows:
[0029]
[0030] m p 92.7-93.8℃; IR (neat) 3691, 2935, 2378, 2110, 1662, 1549, 1371, 744cm -1 , 1 HNMR (400MHz, CDCl 3 )δ7.52(d, J=8.0Hz, 2H), 7.28(s, 2H), 7.27(d, J=9.2Hz, 1H), 7.04(t, J=8.3Hz, 2H), 6.92(s, 2H), 4.38(dt, J=13.8, 7.4Hz, 1H), 3.69(d, J=7.2Hz, 2H), 2.42(s, 3H). 13 C NMR (100MHz, CDCl 3 )δ162.70(d, J=248.7Hz), 144.52, 134.90, 134.21, 131.93(d, J=8.9Hz), 131.59(d, J=3.2Hz), 129.81, 127.47, 116.76(d, J=22.7 Hz), 103.18, 50.89, 21.65. HRMS (ESI)...
Embodiment 3
[0032] Add p-methylaniline allene (30mg, 0.1mmol), ferrous chloride (2.5mg, 0.02mmol), azidotrimethylsilane (17.3mg, 0.15mmol) into a 25mL reaction tube, add 2mL di Chloromethane, stirred and refluxed at 40°C for 40 hours until the raw material allenamine was consumed, then the mixture was concentrated under reduced pressure and purified by chromatography (petroleum ether / ethyl acetate=20 / 1) to obtain a yellow solid product (33.4mg, produced The rate is 98%). The structural formula of the obtained compound is as follows:
[0033]
[0034] m p 83.4-84.8℃; IR (neat) 3463, 2935, 2377, 2112, 1661, 1370, 1172, 744cm -1 , 1 HNMR (400MHz, CDCl 3)δ7.61(d, J=7.4Hz, 2H), 7.35(d, J=17.2Hz, 1H), 7.32(s, 2H), 7.21(d, J=7.5Hz, 2H), 6.88(d, J=7.5Hz, 2H), 4.46(dt, J=13.6, 7.3Hz, 1H), 3.75(d, J=7.2Hz, 2H), 2.49(s, 3H), 2.41(s, 3H). 13 C NMR (100MHz, CDCl 3 )δ144.19,139.46,135.35,134.41,133.04,130.32,129.68,127.49,122.38,102.93,51.01,21.63,21.24.HRMS(ESI)m / zcalcd for C 17 h 19 N 4 o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com