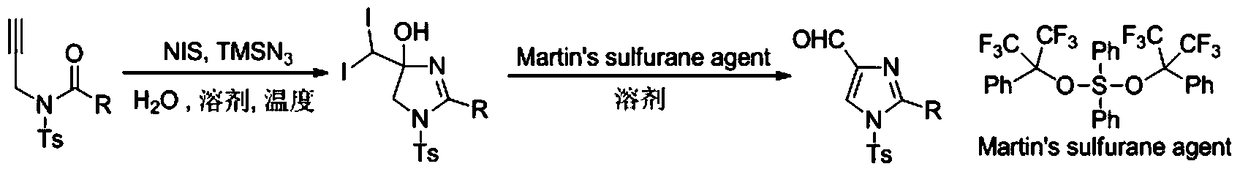
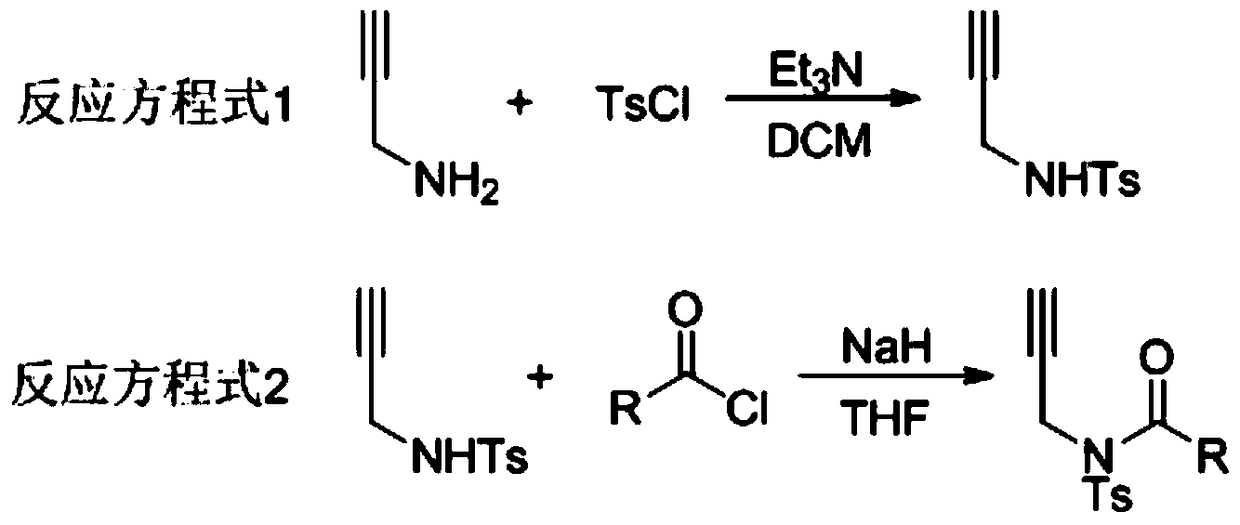
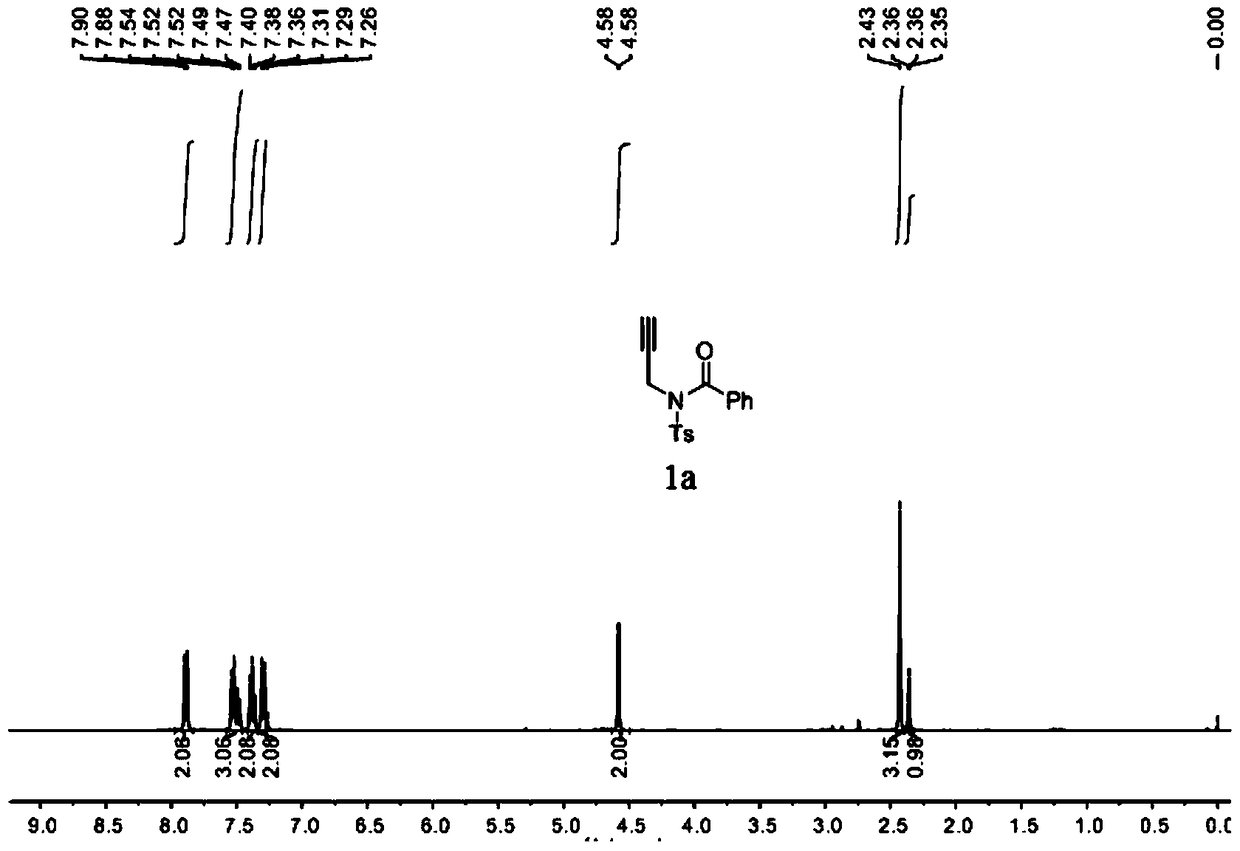
A method for preparing 4-imidazole formaldehyde derivatives by reductive cyclization involving tmsn3
A technology of imidazole formaldehyde and cyclization reaction, applied in the direction of organic chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of propargyl amide 1a: react in a 100mL three-necked reaction flask, vacuumize the reaction tube and replace it with argon three times, then add 10mmol of propargylamine, dissolve it in 50mL of DCM, and then add 20mmol of Et 3 N, then slowly dropwise added 15 mmol of TsCl, stirred at room temperature for 12 hours, extracted with ethyl acetate, and recrystallized with ethanol and petroleum ether to obtain light yellow solid propargyl-p-toluenesulfonamide. The reaction was carried out in a 50mL three-necked reaction flask. After the reaction tube was evacuated and replaced with argon three times, 6mmol of NaH was added, dissolved in 20mL of tetrahydrofuran, and then 5mmol of propargyl p-toluenesulfonamide was weighed, dissolved in 10mL of tetrahydrofuran, Under the condition of ℃, the solution was slowly added dropwise to the reaction system, stirred for 1h, then 7.5mmol of benzoyl chloride was added dropwise, reacted for 3h at room temperature, saturated NH 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com