Synthetic method of alpha-hydroxyl alkenyl azide compound
A synthesis method and hydroxyalkene technology, applied in organic chemistry and other directions, can solve the problems of harsh reaction conditions, narrow substrate range, numerous steps, etc., and achieve the effects of simple steps, simple raw materials, and shortened reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
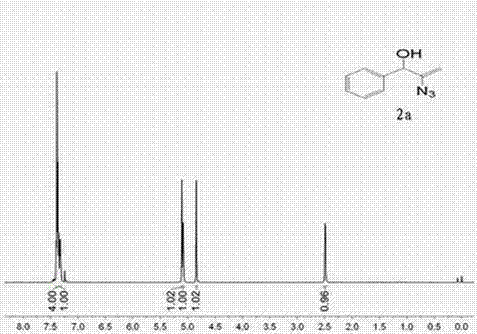
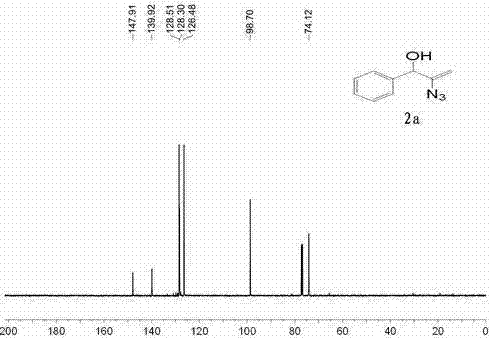
[0025] Example 1 α-Hydroxyalkenyl Azide Derivatives 2a preparation of
[0026]
[0027] Add dimethyl sulfoxide (DMSO) (2 mL), 1-phenyl-2-proparg-1-ol to a 25 mL round bottom flask with a magnetic stirring device 1a (1 mmol), azidotrimethylsilane (TMSN 3 ) (1.5 mmol), put it in an 80°C oil bath, stir well, add silver carbonate (0.1 mmol), and continue stirring. The reaction time is 1h, at this time TLC detects the substrate 1a After disappearing, the reaction solution was poured into water (15 mL), extracted with dichloromethane (3 × 15 mL), and the organic phases were combined. Backwash with water (3 x 20 mL). The organic phase was dried with anhydrous magnesium sulfate, filtered with suction, and then evaporated under reduced pressure to remove the organic solvent, and finally subjected to silica gel column chromatography to obtain a yellow liquid 2a , confirmed by NMR to be α-hydroxyalkenyl azide derivatives 2a , and its yield is 85%.
[0028] Spectral analysis dat...
Embodiment 2
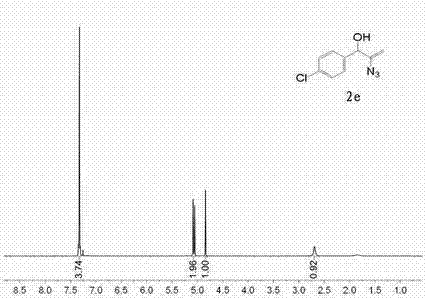
[0030] Example 2 α-Hydroxy alkenyl azide derivatives 2e preparation of
[0031] Replace the 1-phenyl-2-propargyl-1-alcohol in "Example 1" with 1-(p-chlorophenyl)-2-propargyl-1-alcohol, and the reaction conditions change the catalyst in "Example 1" to Silver nitrate (consumption is constant), temperature changes 25 ℃ into, and the reaction times is 8h, and other steps, consumption are all constant. The experimental results are shown in Table 1
[0032]
[0033] Spectral analysis data 2e :
[0034] 1 H-NMR (500 MHz, CDCl 3 ) δ 2.69 (s, 1H), 4.82-4.84 (m, 1H), 5.05 (s, 1H), 5.07-5.09 (m, 1H), 7.30-7.34 (m, 4H); 13 C-NMR (CDCl 3 , 125 MHz) δ 73.5, 98.9, 127.9, 128.6, 134.0, 138.4, 147.8.
Embodiment 3
[0035] Example 3 α-Hydroxyalkenyl Azide Derivatives 2h preparation of
[0036] With 1-(2-furyl)-2-proparg-1-ol instead of 1-phenyl-2-proparg-1-alcohol in "Example 1", the reaction conditions change "Example 1" catalyst into fluorine silver oxide, trimethylsilyl azide was changed to sodium azide, the solvent was changed to N,N -Dimethylformamide (DMF), the reaction time is 5h, and other steps and dosages remain unchanged. The experimental results are shown in Table 1.
[0037]
[0038] Spectral analysis data 2h :
[0039] 1 H-NMR (500 MHz, CDCl3) δ 2.55-2.73 (m, 1H), 4.88-4.91 (d, J = 1.0 Hz, 1H), 5.10-5.16 (m, 2H), 6.34-6.39 (m, 2H), 7.39- 7.42 (m, 1H); 13 C-NMR (CDCl3, 125 MHz) δ 68.2, 99.5, 107.9, 110.5, 142.7, 145.5, 152.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com