Synthesis method for preparing a large amount of alpha-vinyl azide compounds
An azide and synthetic method technology, which is applied in the directions of organic chemistry, sulfide preparation, sulfonic acid amide preparation, etc., can solve the problems of difficult industrial production, harsh reaction conditions, narrow substrate range, etc., and achieves easy modification and application. , The effect of short reaction time and easy processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of α-vinyl azide compound 2a
[0027]
[0028] To a 100 mL round bottom flask equipped with a 3 cm oval magnetic stir bar was added p-tolueneacetylene 1a (50 mmol) followed by dimethyl sulfoxide (DMSO) (50 mL). Then trimethylsilyl azide (75 mmol) and water (100 mmol) were added thereto via a 10 mL syringe. Finally, newly prepared white silver azide (2.5 mmol) was added thereto, and stirred evenly under air condition. Subsequently, the flask was placed in an oil bath at a temperature of 80° C. for the reaction. After 100 min, TLC detected that the substrate disappeared. The round bottom flask was taken out from the oil bath, and after the reaction was cooled to room temperature, the dark brown solution was transferred to a 250 mL beaker, and 80 mL of dichloromethane and 100 mL of water were added thereto for extraction. And the upper aqueous phase was continuously extracted with 50 mL of dichloromethane, and this was repeated three to five times. The o...
Embodiment 2
[0033] Preparation of α-vinyl azide compound 2b
[0034]
[0035] Phenylacetylene 1b was used to replace p-tolueneacetylene 1a in "Example 1", the reaction conditions were changed to 120 min in the reaction time of "Example 1", and other steps and dosages were unchanged. The experimental results are shown in Table 1.
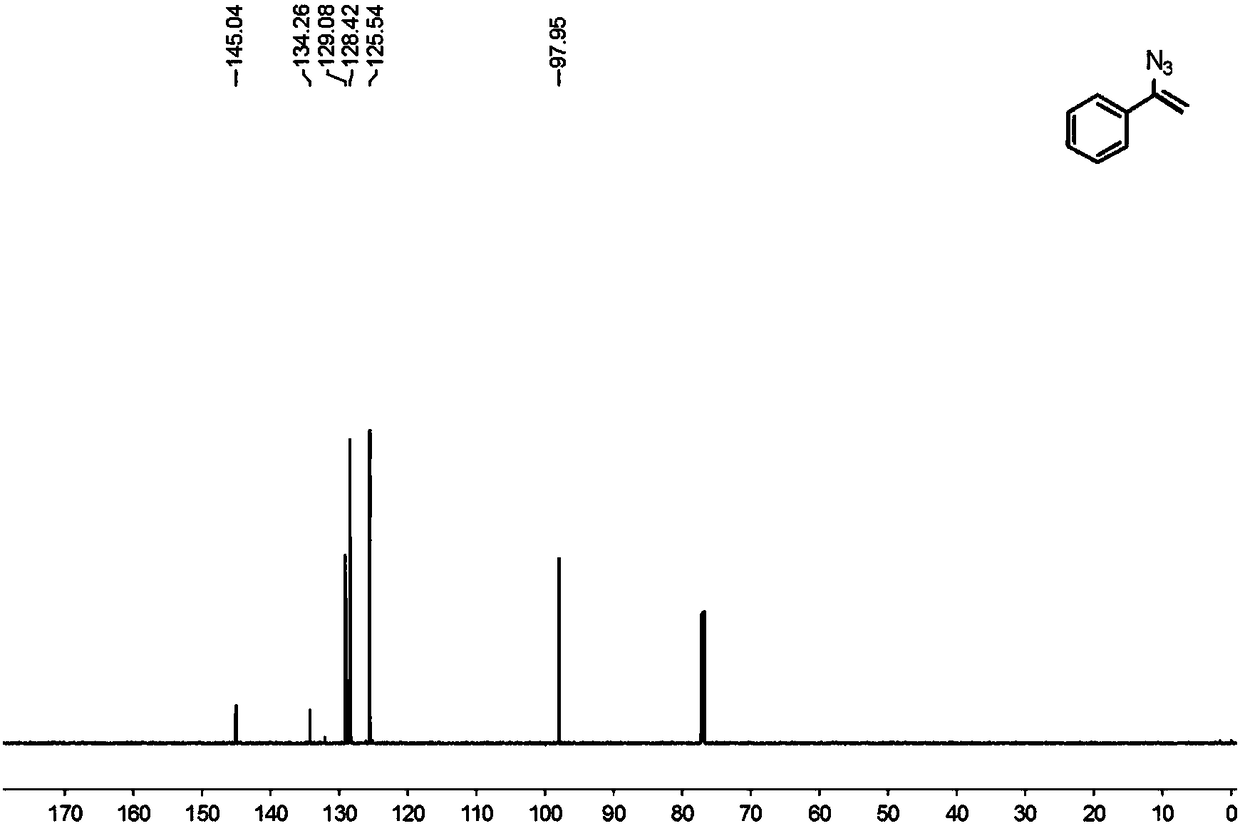
[0036] Spectrum Analysis Data 2b(C 8 h 7 N 3 )
[0037] 1 H NMR (600MHz, CDCl 3 )δ7.61-7.55(m,2H),7.39-7.34(m,3H),5.44(d,J=2.4Hz,1H), 4.97(d,J=2.4Hz,1H);
[0038] 13 C NMR (150MHz, CDCl 3 )δ145.04, 134.26, 129.08, 128.42, 125.54, 97.95.
Embodiment 3
[0040] Preparation of α-vinyl azide compound 2c
[0041]
[0042] Replace p-tolylacetylene 1a in "Example 1" with p-chlorophenylacetylene 1c, and the reaction conditions change the reaction times into 80min in "Example 1", and other steps and consumption are all constant. The experimental results are shown in Table 1.
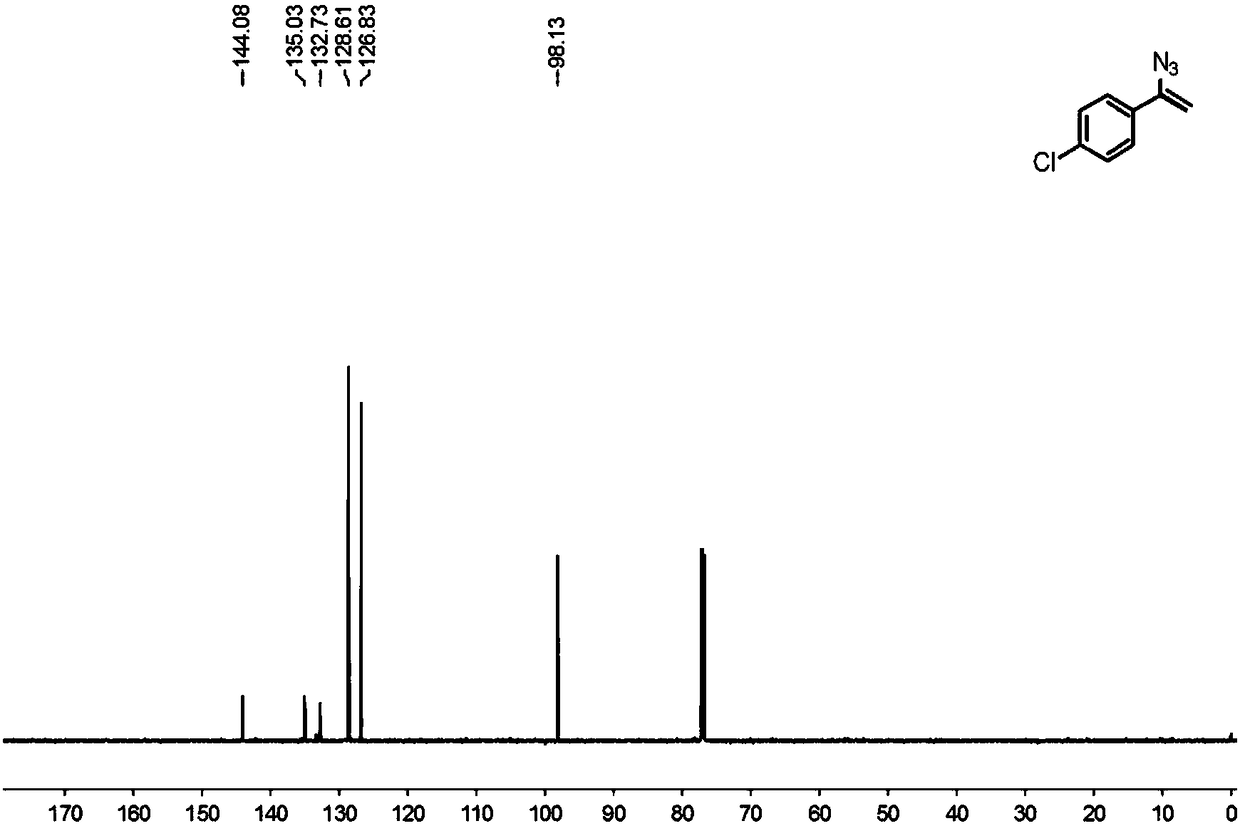
[0043] Spectrum Analysis Data 2c(C 8 h 6 ClN 3 )
[0044] 1H NMR (600MHz, CDCl 3 )δ7.49(d, J=8.4Hz, 2H), 7.32(d, J=9.0Hz, 2H), 5.43(d, J=3.0Hz, 1H), 4.97(d, J=2.4Hz, 1H) ;
[0045] 13 C NMR (150MHz, CDCl 3 )δ144.08, 135.03, 132.73, 128.61, 126.83, 98.13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com