Preparation method of ketamine and synthesis method of intermediate compound
The technology of a compound, ketamine, is applied in the field of drug synthesis, which can solve the problems of acute poisoning, shock or renal failure, easy pollution of the environment by waste iron powder and acid water, easy explosion of nitrating reagents, etc., to achieve mild conditions and high product quality Improved, low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
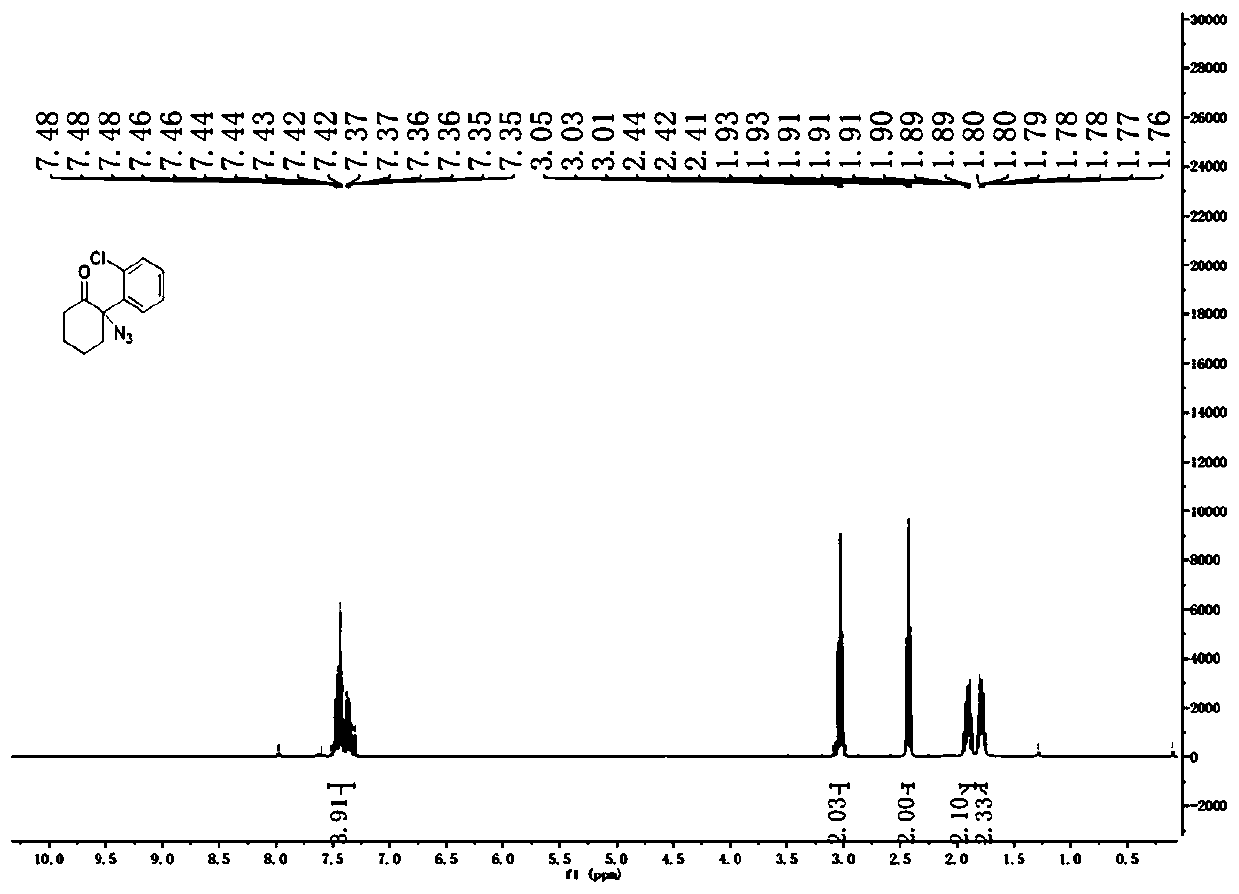
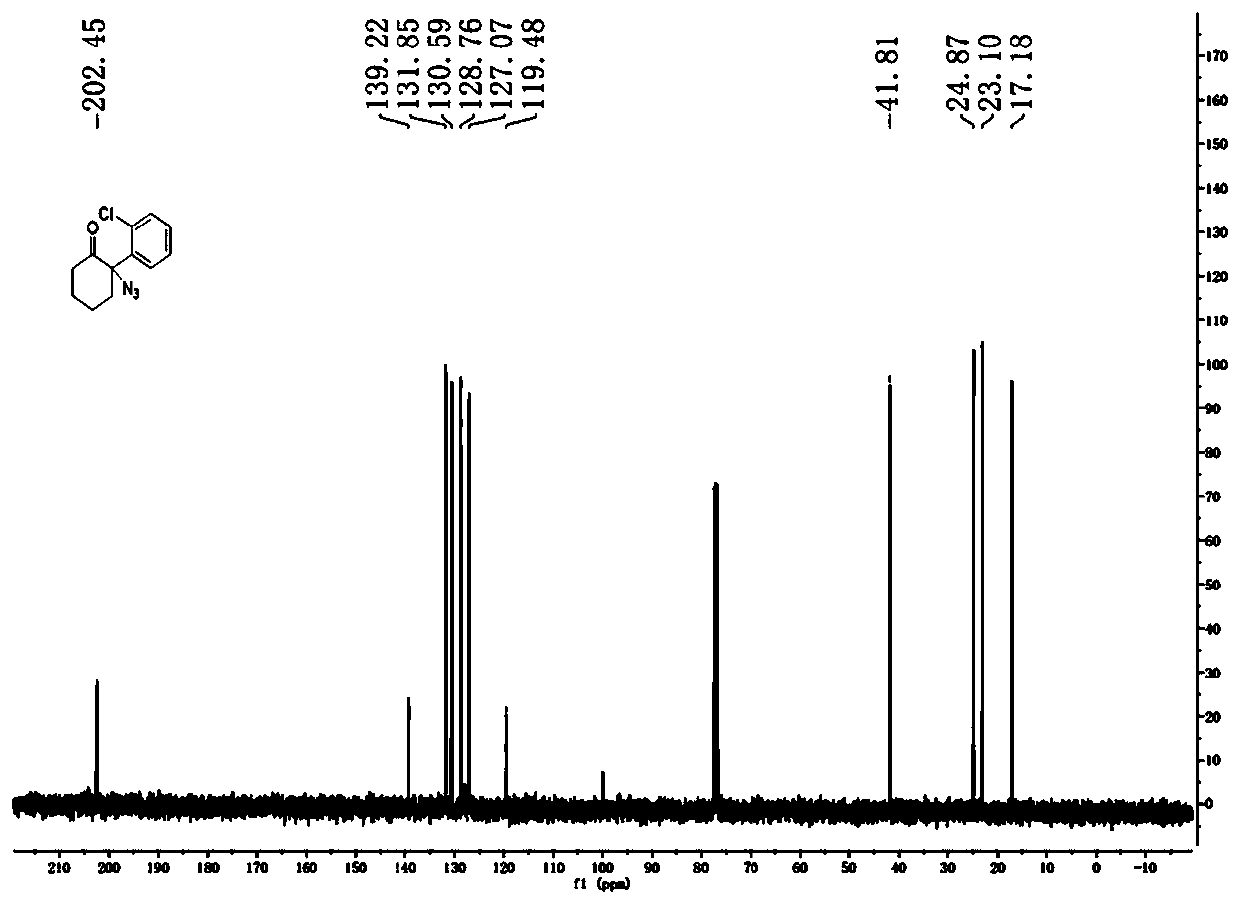
[0042] Embodiment 1: intermediate compound II: the synthesis of 2-o-chlorophenyl-2-azidocyclohexanone
[0043] Dissolve 1-o-chlorophenylcyclohexene (1.92g) in 30mL of dichloromethane, then add PhI(OAc) at -20°C 2 (3.86g) and TMSN 3 (1.75g), kept at -20°C for 16h. After the reaction was completed, the temperature of the system was raised to room temperature, the reaction solution was washed with water, and extracted with ethyl acetate, the organic phases were combined, and the organic phase was washed with anhydrous Na 2 SO 4 After drying and concentration, column chromatography (petroleum ether: ethyl acetate = 20:1) gave 2-o-chlorophenyl-2-azidocyclohexanone (1.78 g, yield 71%) as a colorless oily liquid. 1 H NMR (400MHz, CDCl3 ):δ=1.76-1.80(m,2H),1.81-1.93(m,2H),2.41-2.44(m,2H),3.01-3.05(m,2H),7.34-7.48(m,4H); 13 C NMR (100MHz, CDCl 3 ): δ=17.18, 23.10, 24.87, 41.87, 119.48, 127.07, 128.76, 130.59, 130.74, 131.85, 139.22, 202.45.
[0044] Compound (II) was detected acc...
Embodiment 2
[0045] Embodiment 2: intermediate compound II: the synthesis of 2-o-chlorophenyl-2-azidocyclohexanone
[0046] Dissolve 1-o-chlorophenylcyclohexene (19.2g) in 300mL of dichloromethane, then add iodosobenzene (26.4g) and 1-(azido)-1,2-benzene at -22°C Iodosyl-3(1H)-one (43.4g) was kept at -22°C for 17h. After the reaction was completed, the temperature of the system was raised to room temperature, the reaction solution was washed with water, and extracted with ethyl acetate, the organic phases were combined, and the organic phase was washed with anhydrous Na 2 SO 4 After drying and concentration, column chromatography (petroleum ether: ethyl acetate = 20:1) gave 2-o-chlorophenyl-2-azidocyclohexanone (20.4 g, yield 82%) as a colorless oily liquid.
Embodiment 3
[0047] Embodiment 3: intermediate compound II: the synthesis of 2-o-chlorophenyl-2-azidocyclohexanone
[0048] Dissolve 1-o-chlorophenylcyclohexene (19.2 g) in 300 mL of dichloromethane, then add bis(trifluoroacetoxy) iodobenzene (58 g) and 1-(azido)- 3,3-Bistrifluoromethyl-1,3-dihydro-1,2-benzoiodooxolane (45.4g) was kept at -18°C for 13h. After the reaction was completed, the temperature of the system was raised to room temperature, the reaction solution was washed with water, and extracted with ethyl acetate, and the organic phases were combined, and the organic phase was washed with anhydrous MgSO 4 After drying and concentration, column chromatography (petroleum ether: ethyl acetate = 20:1) gave 2-o-chlorophenyl-2-azidocyclohexanone (21.6 g, yield 87%) as a colorless oily liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com