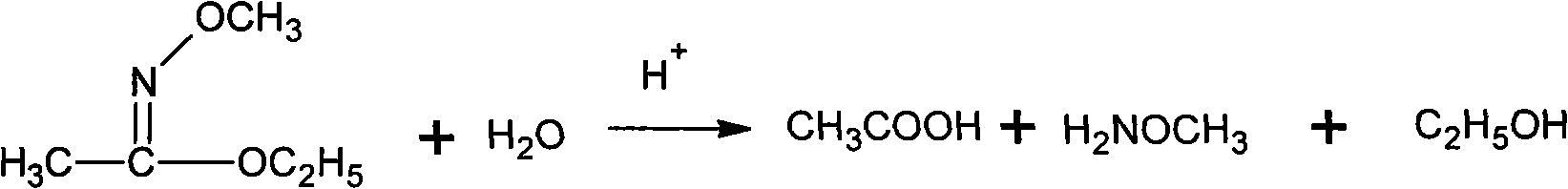
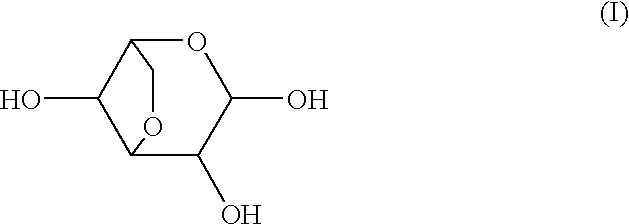
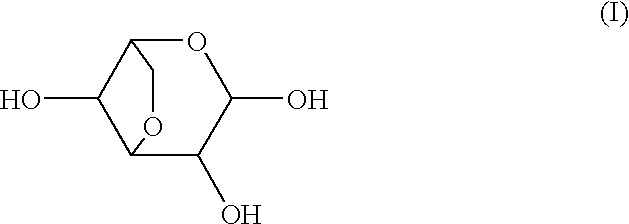
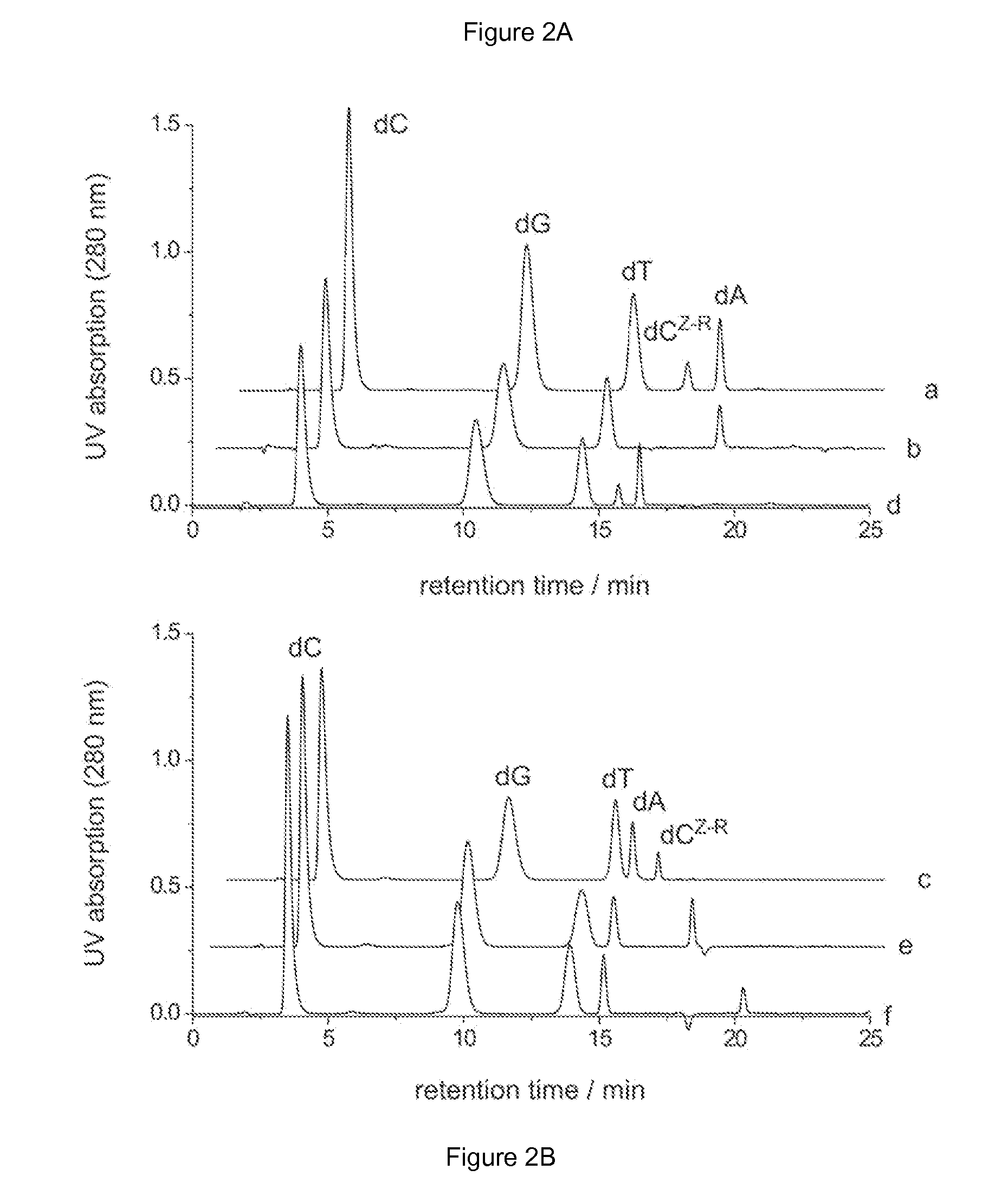
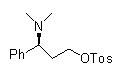
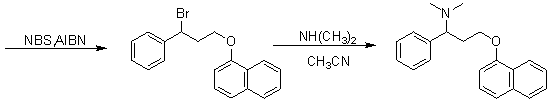
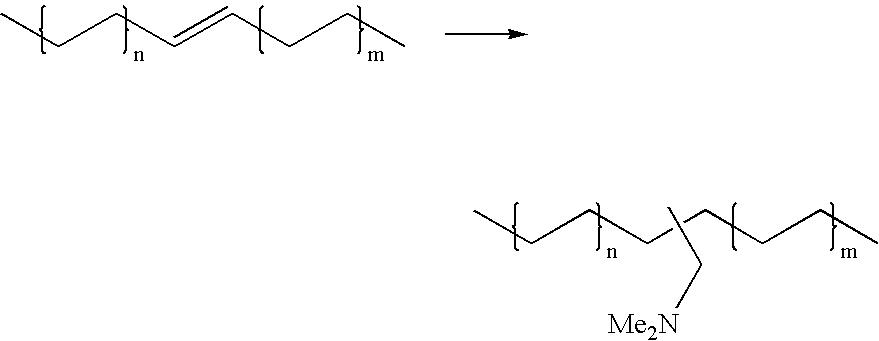
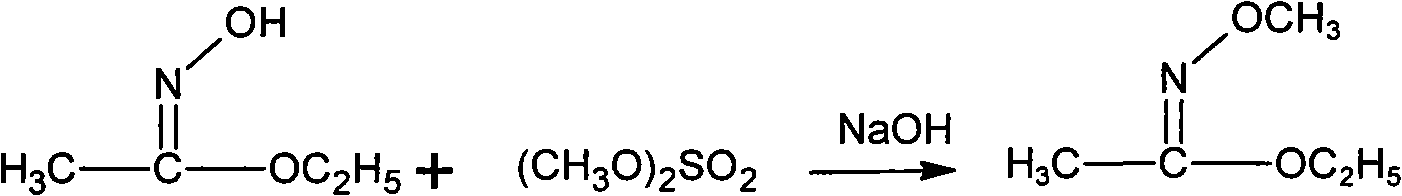Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
653 results about "Transmethylation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Transmethylation is a biologically important organic chemical reaction in which a methyl group is transferred from one compound to another. An example of transmethylation is the recovery of methionine from homocysteine. In order to sustain sufficient reaction rates during metabolic stress, this reaction requires adequate levels of vitamin B₁₂ and folate. Methyl tetrahydrofolate delivers methyl groups to form the active methyl form of vitamin B₁₂ that is required for methylation of homocysteine. Deficiencies of vitamin B₁₂ or folate cause increased levels of circulating homocysteine. Elevated homocysteine is a risk factor for cardiovascular disease and is linked to the metabolic syndrome (insulin insensitivity).
Synthetic technology for pyraclostrobin
ActiveCN104211641AFormation reaction is easy to controlSmooth responseOrganic chemistryMethylanilineChlorobenzene
The invention concretely relates to a synthetic technology for pyraclostrobin. The synthetic technology comprises: firstly performing cyclization to obtain 1-(4-chlorophenyl)-pyrazol-3-one, oxidizing the pyrazol ring under the effect of an oxidant to generate 1-(4-chlorophenyl)-3-hydroxypyrazole, then using 2-nitrobenzyl bromide to performing etherification to generate 1-(4-chlorophenyl)-3-[2-(nitrophenyl)methoxy]-1H-pyrazole, then using a reducing agent to perform nitro reducing, so as to generate N-hydroxyl-2-[N'-(4-chlorophenyl)pyrazol-3'-yloxymethyl]aniline, then using ClCO2CH3 to perform N-acylation reaction to generate methyl N-hydroxyl-N-2-{[N'-(4-chlorophenyl)pyrazol-3'-yloxymethyl]phenyl}formate, and finally performing hydroxyl methylation under an alkaline condition to generate pyraclostrobin. The technology enables all operations in the pyraclostrobin preparation process to be relatively controllable, helps to improve the stability of the preparation process and improve the product yield, successfully employs low-cost reagents and substantially reduces production cost, and also the employed reagents are relatively small in toxicity, is relatively beneficial for environment protection, and has no corrosivity on plastic pipes, so that the production safety is improved.
Owner:SHANDONG KANGQIAO BIO TECH CO LTD
Medicinal composition as immunosuppressant
InactiveUS20070149597A1Easy to optimizeSimple compositionBiocideOrganic chemistryHMG-CoA reductaseMethyl group
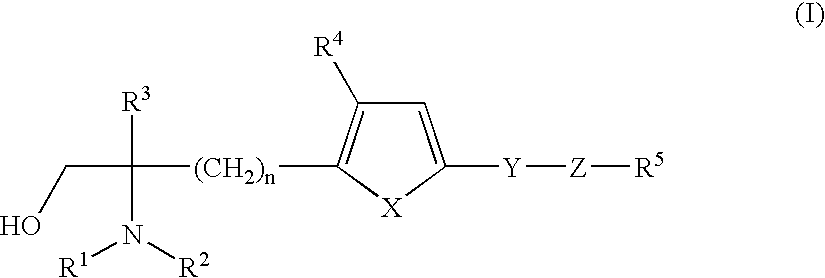
A pharmaceutical composition useful as a preventive or therapeutic agent for immune-related diseases. The pharmaceutical composition includes at least HMG-COA reductase inhibitor and at least one amino alcohol compound having the following formula (I), a pharmacologically acceptable salt thereof, or a pharmacologically acceptable ester thereof wherein R1 and R2 each represents hydrogen; R3 represents lower alkyl or hydroxymethyl; R4 represents hydrogen, alkyl, alkoxy or halogen; R5 represents hydrogen, halogeno, cyclohexyl or phenyl; X represents vinylene (CH═CH), oxygen, sulfur or methylated nitrogen; Y represents a single bond, oxygen, sulfur or carbonyl; Z represents a single bond or C1-C8 alkylene; n is 2 or 3.
Owner:DAIICHI SANKYO CO LTD
Zwitterionic compounds and use thereof
ActiveUS20090054521A1Lack of propertyReduce molecular weightOrganic active ingredientsBiocideMedicineZwitterion
Owner:EVONIK OPERATIONS GMBH
Compositions for the treatment of hepatitis C and methods for using compositions for the treatment of hepatitis C
InactiveUS20060229293A1Treating hepatitis CImprove the level ofBiocideCarbohydrate active ingredientsMetaboliteChronic hepatitis
The present invention pertains to a composition comprising Ibogaine, an indole alkaloid, its active salts and its principal metabolite noribogaine, a demethylated form of ibogaine, for the treatment of hepatitis C and hepatitis C related complications, administered in single or multiple dose regimens effective to reduce somatic complaints, liver enzyme values and viral load caused by chronic hepatitis C in patients, and methods of using the same.
Owner:ADDICTION RES INST INC
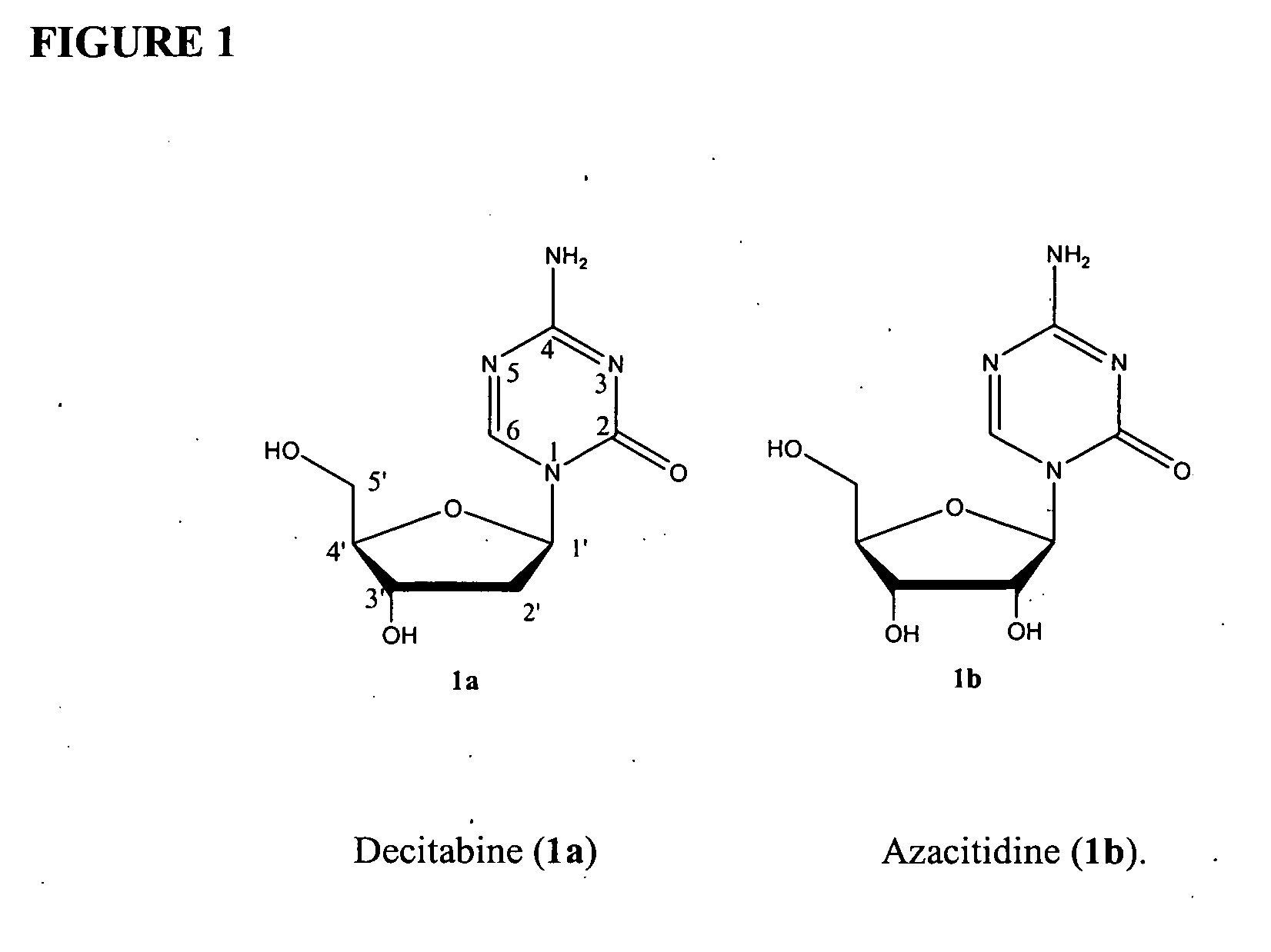
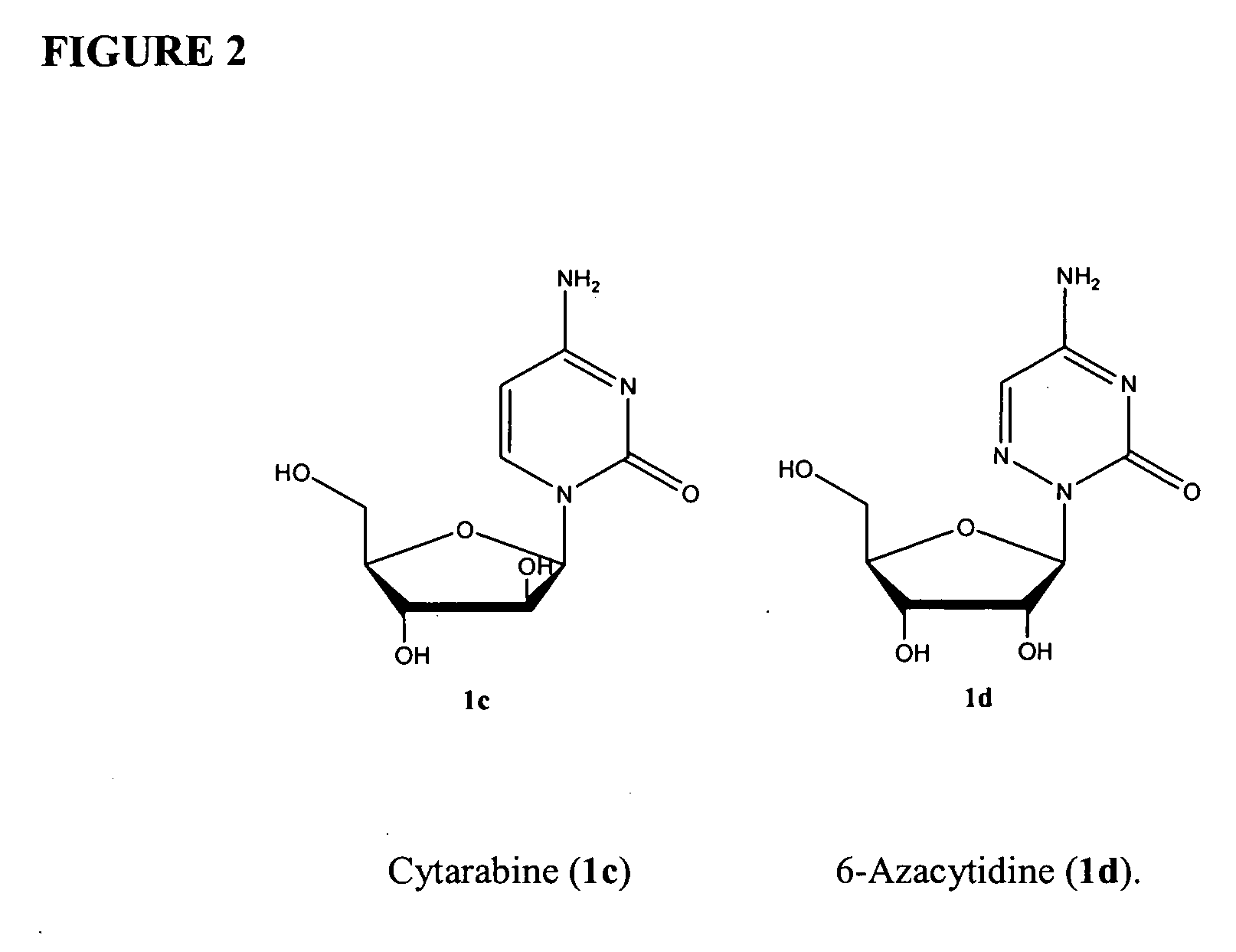
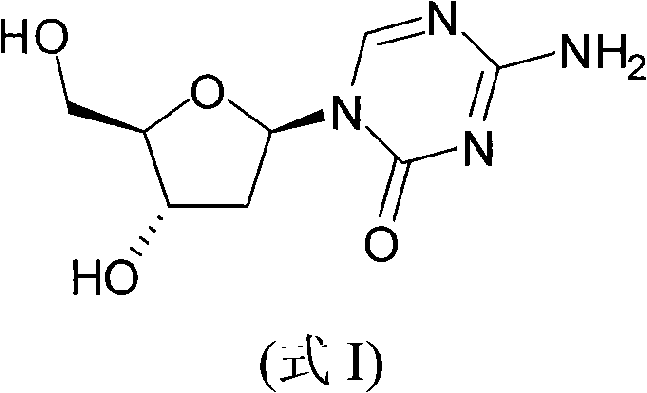
Azacytosine analogs and derivatives
InactiveUS20060205687A1Decrease electrophilicityBiocideSugar derivativesAbnormal tissue growthPyrimidine analogue
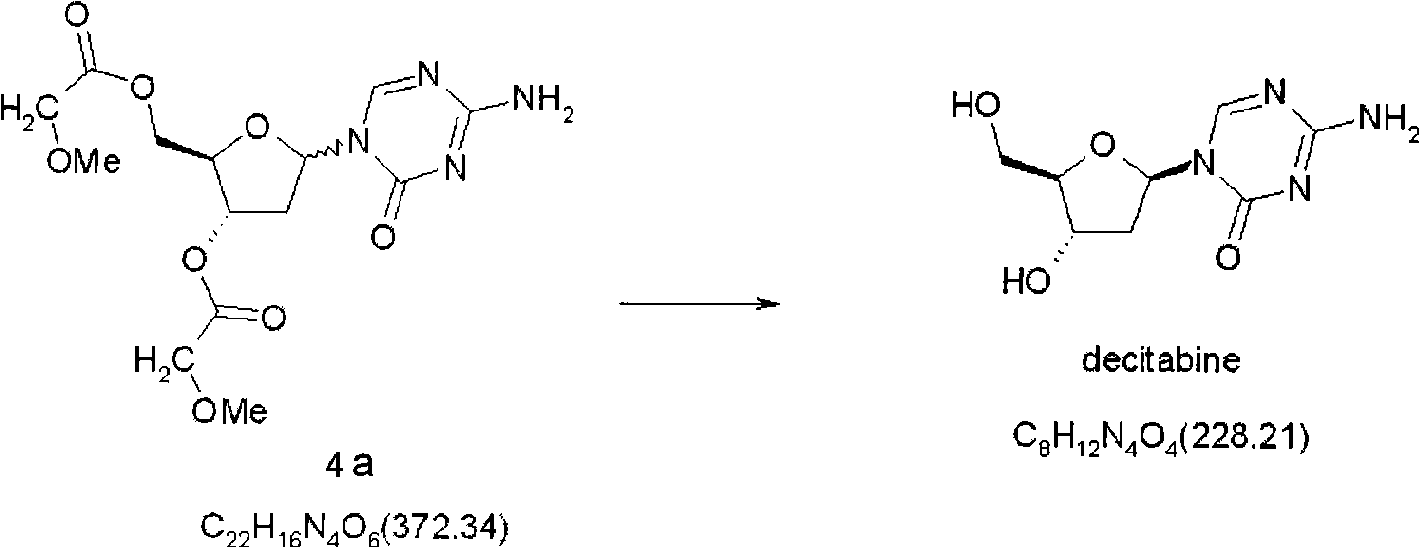
Compounds and compositions of azacytosine analogs and derivatives are provided. In one aspect of the invention, analogs or derivatives of decitabine and azacitidine are provided with modification at the 2-, 4-, or 6-position of the triazine ring, at the 1′-6′position of the ribose ring, or combinations thereof. Methods of using, synthesizing and manufacturing these analogs and derivatives are also provided. These compounds can be formulated into pharmaceutical compositions that can be used for treating any disease associated with aberrant DNA methylation, or a disease or condition that is sensitive to the treatment with decitabine or azacitidine, such as hematological disorders, tumors and cancers.
Owner:SUPERGEN
Process for the synthesis of biologically active oxygenated compounds by dealkylation of the corresponding alkylethers
ActiveUS7253324B1Easy to handleEasy to prepareCarboxylic acid nitrile preparationOrganic compound preparationDimethylaniline N-oxideOxyresveratrol
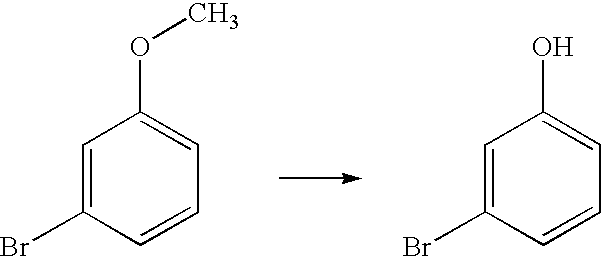
Alkoxy aromatic compounds are conveniently dealkylated to the corresponding phenolic compounds by treatment with aluminum chloride / N,N-dimethyl aniline complex. Aromatic poly O-demethylation is a unique feature of this invention. This process is applicable to the manufacture of polyphenols such as Resveratrol, Oxyresveratrol, Gnetol.
Owner:SAMI LABS LTD
Manufacture of xylenes using reformate
A process is provided for the production of xylenes from reformate. The process is carried out by methylating under conditions effective for the methylation, the benzene / toluene present in the reformate outside the reforming loop, to produce a resulting product having a higher xylenes content than the reformate. Greater than equilibrium amounts of para-xylene can be produced by the process.
Owner:EXXONMOBIL CHEM PAT INC
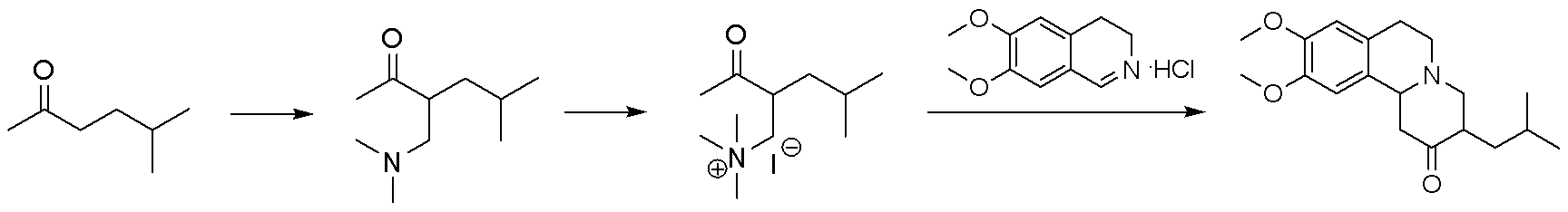
Method for synthesizing tetrabenazine
The invention discloses a method for synthesizing tetrabenazine. The method comprises: step one, using a dimethylamine aqueous solution and a formaldehyde aqueous solution as initial materials to be reacted1 to obtain tetramethyl methane diamine; step two, dissolving the tetramethyl methane diamine obtained in the step one in an organic solvent, dropwise adding acetyl chloride and 5- methyl-2-hexanone, and performing amine methylation to obtain an intermediate 3-[(dimethyl amino) methyl]-5-methyl-2-hexanone; and step three, reacting the 3-[(dimethyl amino) methyl]-5-methyl-2-hexanone obtained in the step two with 6,7-dimethoxy-3,4-dihydroisoquinoline hydrochloride to obtain the tetrabenazine. According to the method for synthesizing tetrabenazine, the cheap and accessible materials are used as the initial materials, imine salts serve as amine methylation reagents to perform amine methylation reaction on the 5- methyl-2-hexanone, and accordingly, the region-selectivity of chemical reactions is improved; and water serves as a reaction solvent to prepare the tetrabenazine, so that the operation is simple and convenient, no complex post-processing process exists, and good industrial application prospects are provided.
Owner:JIANGSU JIMING PHARMA TECH
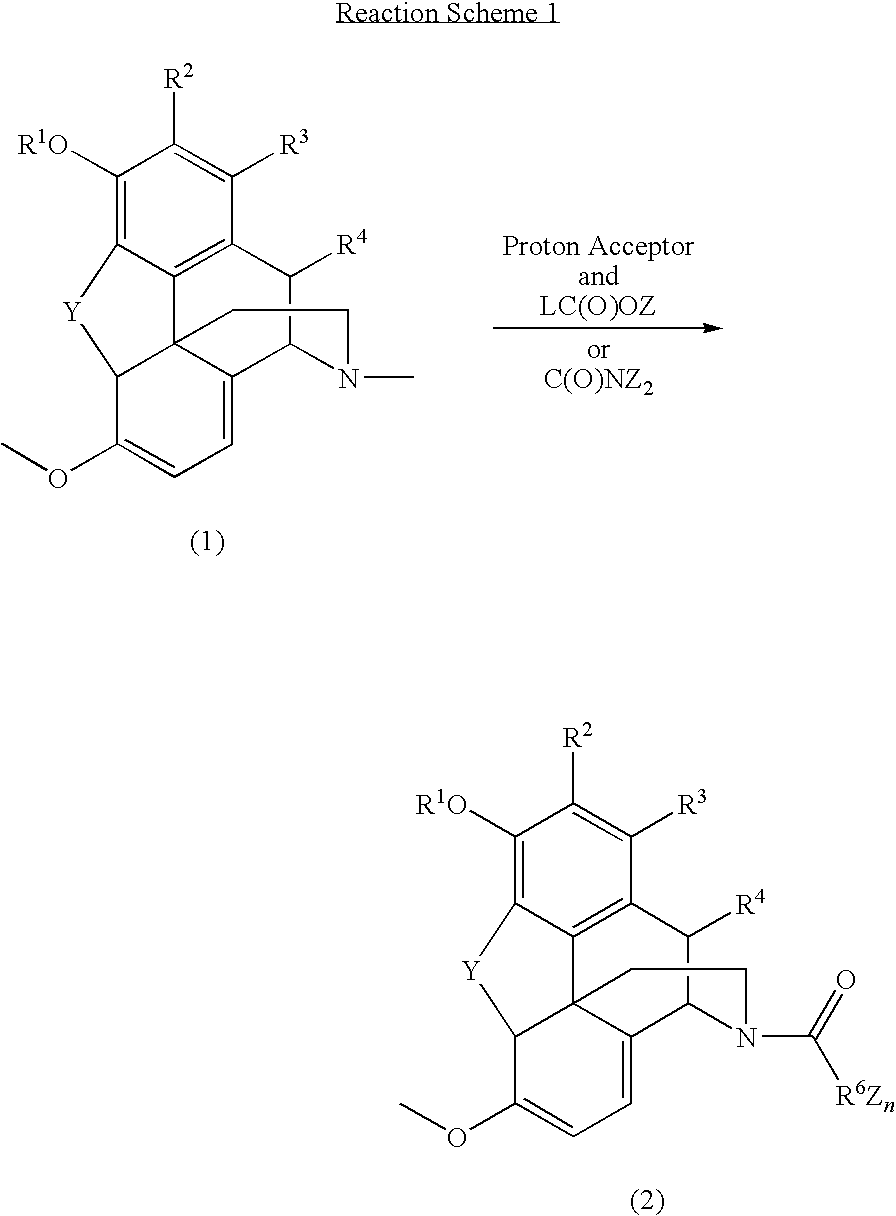
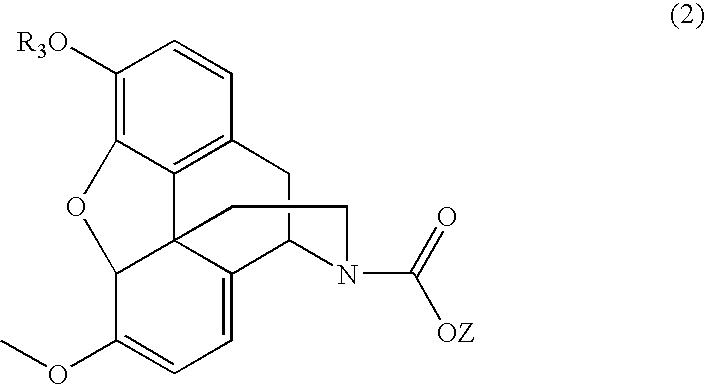
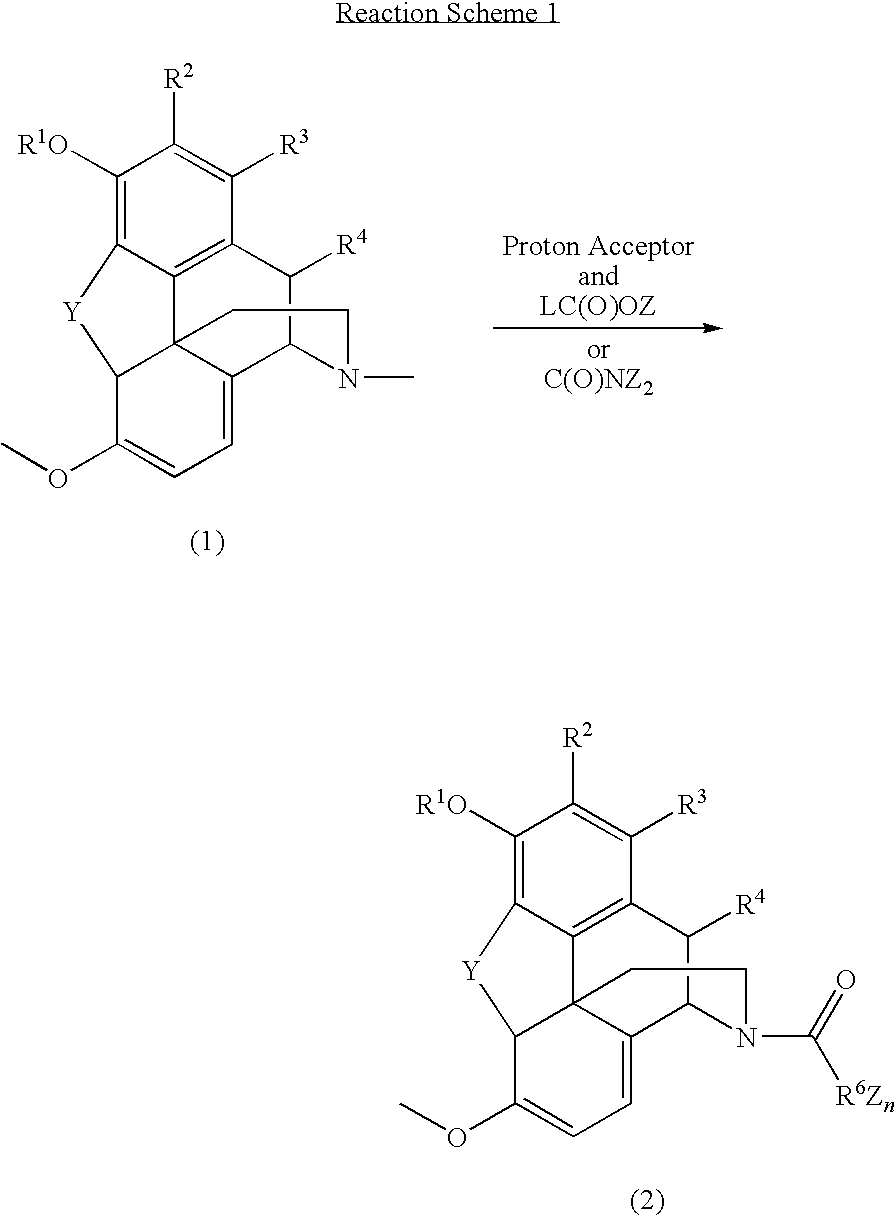
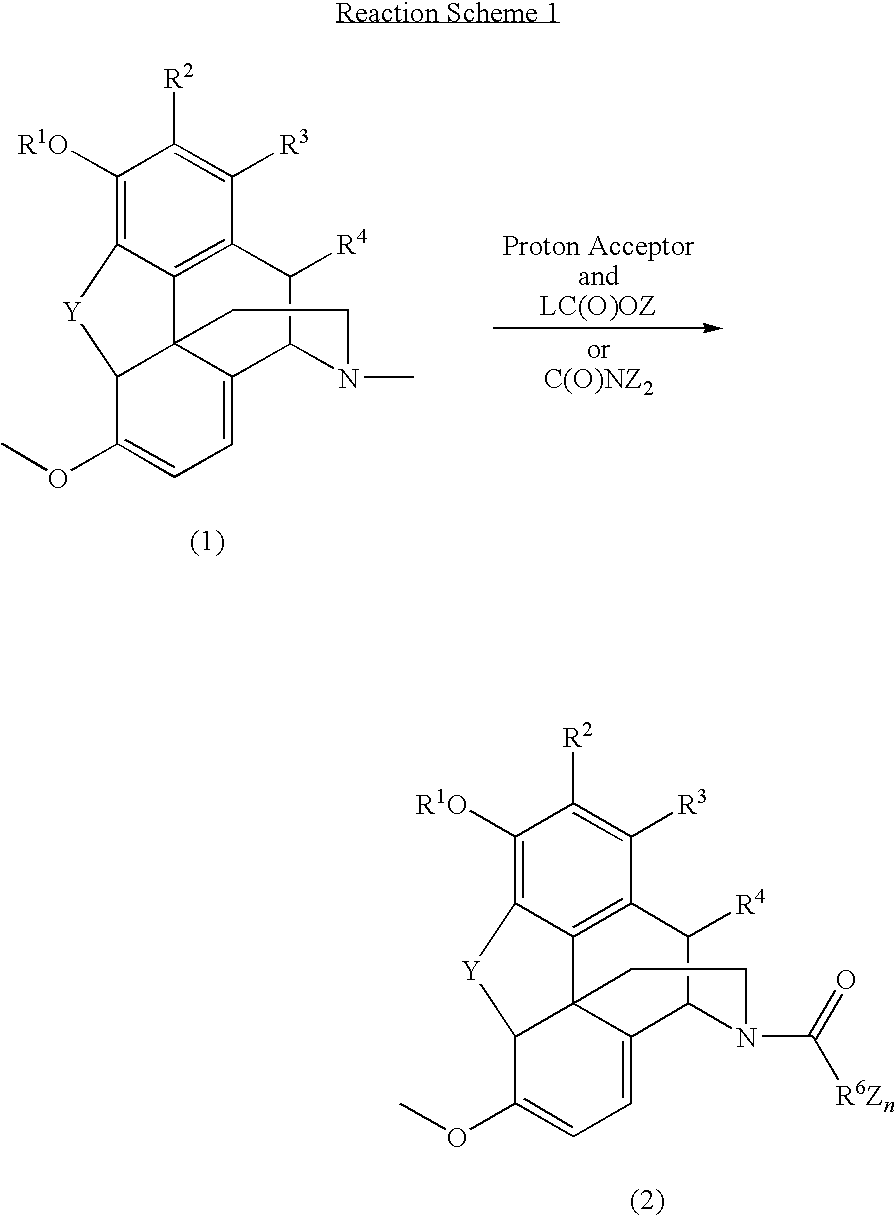
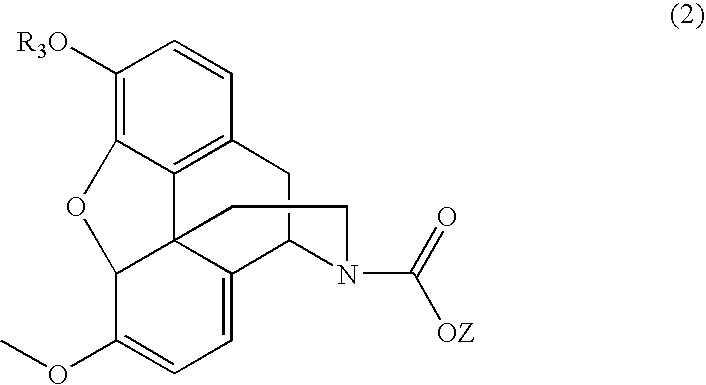
N-demethylation of N-methyl morphinans
The present invention provides a synthetic process for the N-demethylation of N-methyl morphinans. In particular, the invention provides improved synthetic methods for the preparation of N-demethylated morphinan compounds that may be employed as starting materials, for example, commonly available N-methyl opiates such as oripavine and thebaine, and C(3)-protected hydroxy derivatives of oripavine.
Owner:SPECGX LLC
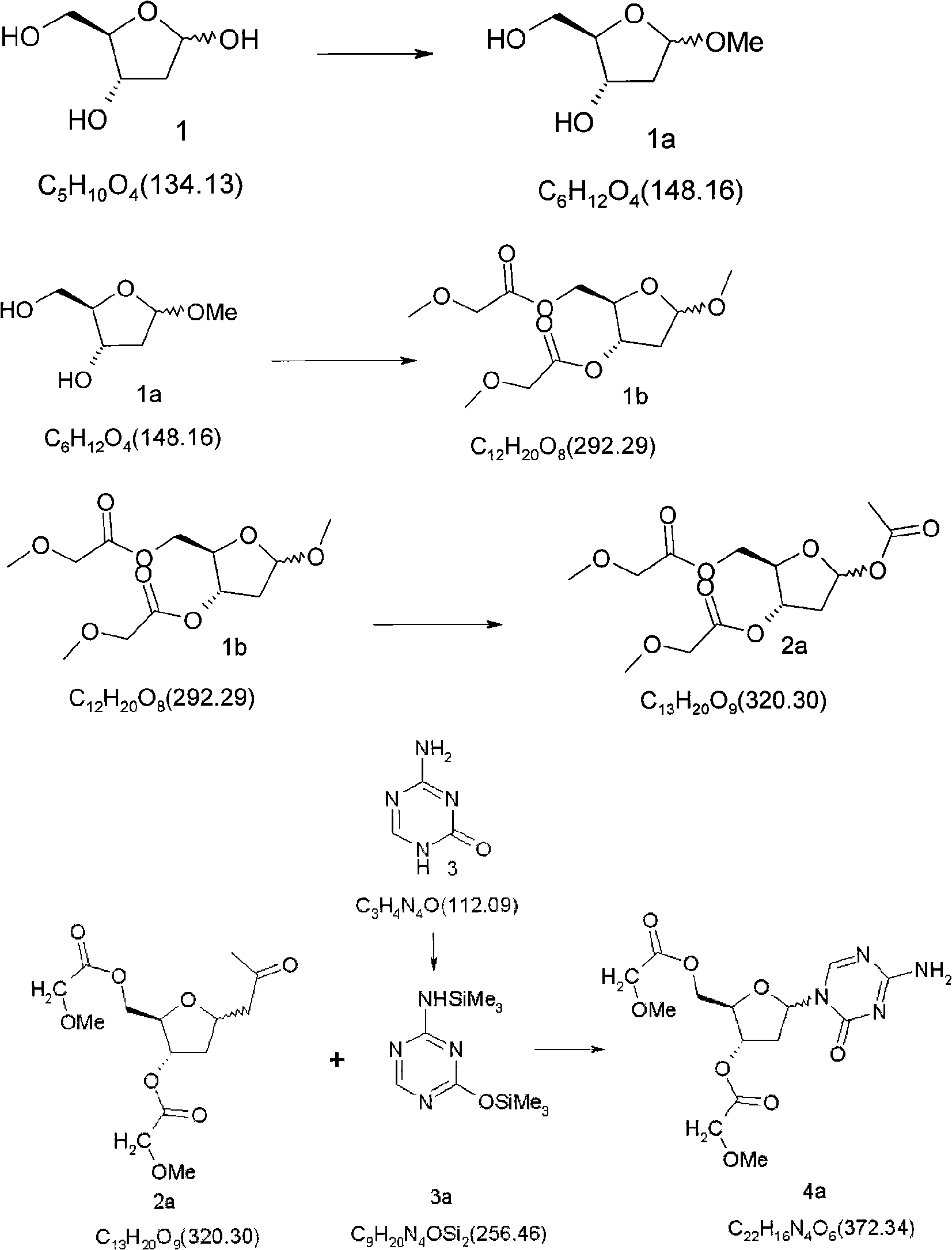
Synthetic process of decitabine
The invention relates to a method for preparing Decitabine. The particular proposal for solving the technical problem is as follows: 2-deoxidtion-D-ribose, 10 percent of HCL methanol solution, methoxyacetic acetic anhydride, HMDS, acetic anhydride, tri-silicyl tri-fluorine methane sulfonic acid ester, acetic acid amine, etc. are adopted as raw materials to synthesize the Decitabine; the target product of the Decitabine is obtained through the five steps of reactions, namely, methylation, acylation, trimethyl silication, ammoniation and deacylation with a total yield of above 18.4 percent and a product purity of above 99.7 percent.
Owner:GUIZHOU UNIV
Transalkylation of methylated aromatic hydrocarbon-enriched fractions in c8 aromatic hydrocarbon production
InactiveUS20120271071A1Improve performanceLess valueDistillation purification/separationLiquid carbonaceous fuelsAromatic hydrocarbonOrganic chemistry
Methods are disclosed for producing C8 aromatic hydrocarbons. Representative methods comprise (a) fractionating an aromatic hydrocarbon containing feed stream (e.g., a feed stream comprising C9 and / or C10 aromatic hydrocarbons), to provide at least one methylated aromatic hydrocarbon-enriched fraction; and (b) reacting at least a portion of the at least one methylated aromatic hydrocarbon-enriched fraction in a transalkylation reaction zone to provide a transalkylation effluent comprising the C8 aromatic hydrocarbons. The presence of a methylated aromatic hydrocarbon-enriched fraction in the inlet stream to the transalkylation reaction zone provides a number of advantages as described herein.
Owner:UOP LLC
Enantiomers of S-adenosyl-l-methionine
Enantiomers of S-adenosyl-l-methionine, their stable salts and their uses are described. These compositions possess potent activity in treating various conditions involving hypomethylation and transulfuration reactions and are valuable for use as active constituents in pharmaceutical compositions.
Owner:HEBERT ROLLAND F
Method for preparing ticagrelor key intermediate
InactiveCN106279095AReduce pressure on environmental protectionSuitable for large-scale industrial productionOrganic chemistryBulk chemical productionChemical synthesisPalladium on carbon
The invention relates to a chemical synthesis method of ticagrelor key intermediate 2-[[(3aR, 4S, 6R, 6aS)-6-aminotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxolane-4-yl] oxy]ethanol (a key intermediate A). The method comprises the following steps: taking D-ribose as a raw material, and carrying out ten chemical reaction steps of 1-locus methylation and 2,3-loci isopropylidene protection, 4-locus derivatization, iodination, furan ring-opening, hydroxylamine reaction, palladium on carbon catalytic hydrogenation, amino Cbz protection, hydroxy protection, sodium borohydride reduction ester, Cbz removal protection and the like, thereby obtaining the key intermediate A. The raw materials are cheap and readily available, the preparation process is high in operability, steps of optical resolution, chiral induction and the like are avoided, the total yield is relatively high, and the product quality is better; particularly due to the use of sodium borohydride reduction ester, the preparation cost of ticagrelor is greatly reduced; and the method is suitable for large-scale industrial production.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
Method for realizing selective N-methylation of primary amine
InactiveCN103172523ANo harmImprove economyOrganic compound preparationSulfonic acid amide preparationIridiumNitrogen gas
The invention relates to a method for realizing selective N-methylation of primary amine, which comprises the following steps: in a nitrogen gas protective atmosphere or in the air, adding a primary amine derivative, a metal iridium or ruthenium complex, methanol and alkali into a reaction vessel; reacting the reaction mixture at 100-150 DEG C for several hours, and then cooling to room temperature; and performing rotary evaporation to remove the solvent, and then performing column separation to obtain a target compound. Compared with the prior art, the reaction shows the following remarkable advantages: 1) the methanol is used as an alkylation agent, thereby avoiding the use of virulent haloalkane and dimethyl sulfate; 2) the reaction only generates water as the byproduct, thereby causing no environmental hazard; 3) the reaction is high in atom economy; 4) the reaction shows absolute selectivity, and the reaction only generates mono-methyl products and generates no bis-methyl products; and 5) the reaction shows wide substrate universality and is effective for various primary amine derivatives.
Owner:NANJING UNIV OF SCI & TECH
Synthetizing method of lacosamide
InactiveCN103113256AHighlight substantive featuresSignificant progressOrganic compound preparationCarboxylic acid amides preparationPtru catalystAmmonium chloride mixture
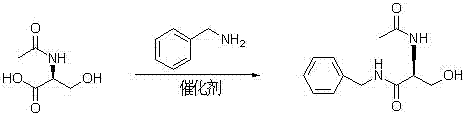
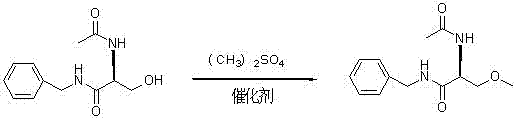
The invention provides a synthetizing method of lacosamide. The method comprises the steps of: based on D-serine as a raw material, performing an acylation reaction with acetic anhydride and then performing a condensation reaction with benzylamine; and finally, performing a methylation reaction with dimethyl sulfate, thereby obtaining lacosamide, wherein N,N' dicyclohexylcarbodiimide (DCC) or N,N' carbonyl diimidazole (CDI) is used as a catalyst in the condensation reaction; and a phase transfer catalyst including triethyl benzyl ammonium chloride (TEBA), tetrabutylammonium chloride (TBAC), tetrabutylammonium bromide (TBAB) or tetrabutylammonium hydrogen sulfate (TBAHS) is adopted in the methylation reaction. The method has the advantages of being simple in synthetizing process, moderate in reaction condition, simple in after-treatment, high in yield and high in product purity.
Owner:SUZHOU HONGRUI MEDICAL TECH
Method for synthesizing methoxamine hydrochloride
ActiveCN101357895AReduce usageImprove the operating environmentOrganic chemistryHalohydrocarbonHydroxylamine Hydrochloride
The invention provides a method for synthesizing methoxyamine hydrochloride and includes the following steps: ethyl acetate and hydroxylamine hydrochloride are added into a reaction vessel and 10 to 30 percent of sodium hydroxide solution is instilled for oximation reaction; then dimethyl sulfate is instilled, and at the same time sodium hydroxide solution with the mass fractions of 10 to 30 percent is instilled for methylation reaction; cold water is added after the temperature is decreased and a halohydrocarbon solvent is adopted for extraction; pressure is reduced under the temperature of 30 to 50 DEG C to recover the halohydrocarbon solvent and the product obtained is added into inorganic acid solution for hydrolysis reaction; after the hydrolysis is over, hydrochloride is used to obtain the product methoxyamine hydrochloride. The synthetic method which is simple improves the operation environment and increases the yield ratio.
Owner:JIANGSU QINGQUAN CHEM CO LTD
Medicinal compositions
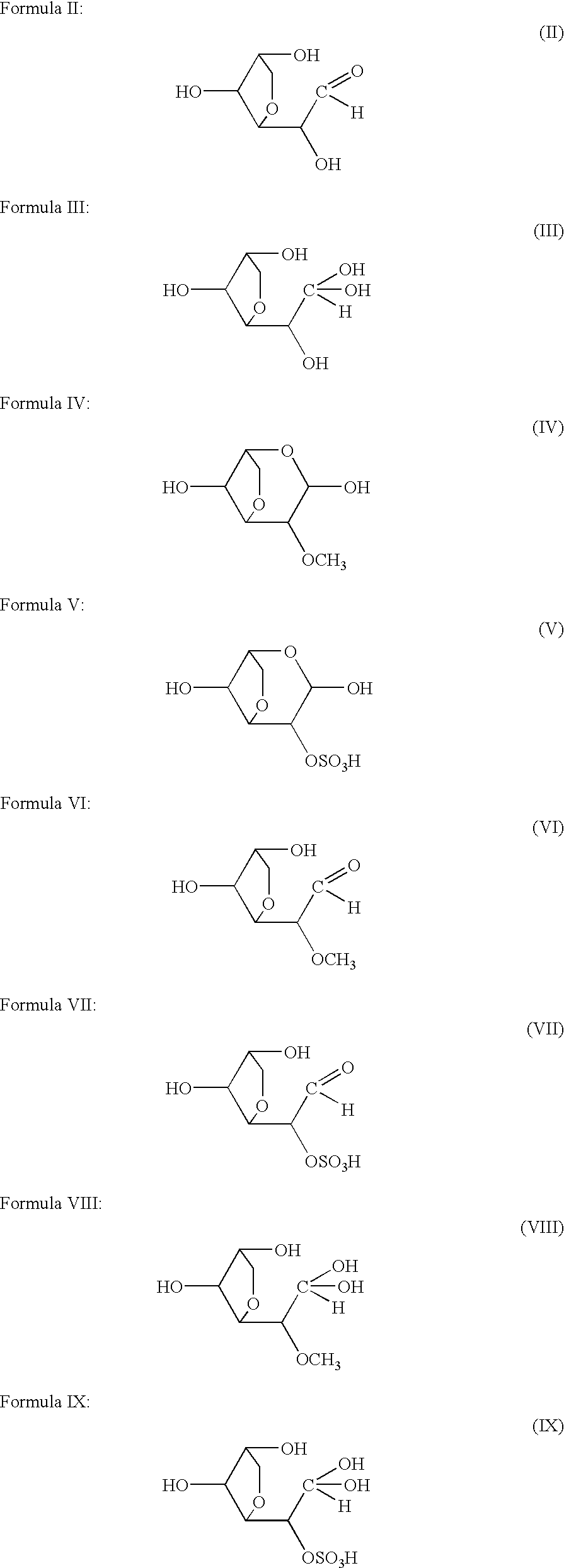
Medicinal compositions for treating or preventing diabetes, rheumatoid, diseases wherein inflammation should be inhibited, diseases wherein alpha-glycosidase should be inhibited, diseases wherein the synthesis of prostaglandin should be inhibited, diseases wherein endotoxin shock should be inhibited, diseases wherein the production of interleukin should be inhibited, diseases wherein the production of heme oxygenase should be induced, and diseases wherein the production of tumor necrosis factor or carcinogenesis should be inhibited, which contain as the active ingredient at least one compound selected from the group consisting of 3,6-anhydrogalactopyranose represented by formula (I), its aldehyde, its hydrate and 2-O-methylated derivatives and 2-O-sulfated derivatives thereof.
Owner:TAKARA HOLDINGS
Preparation method of 2-substituted arylethenyl-N-methylated quinoline derivative and application of 2-substituted arylethenyl-N-methylated quinoline derivative in preparation of drug for treating Alzheimer disease
ActiveCN103333156AGood anticholinergic activityAnticholinergic activity maintainedNervous disorderOrganic chemistryDiseaseMorpholine
The invention relates to the field of medicinal chemistry and pharmacotherapeutics and provides a 2-substituted arylethenyl-N-methylated quinoline derivative, a synthesis method thereof and an application of the 2-substituted arylethenyl-N-methylated quinoline derivative to the preparation of a drug for treating Alzheimer disease. The 2-substituted arylethenyl-N-methylated quinoline derivative has the chemical formula shown in the specification, wherein R1 in the formula is hydrogen, methylpiperazine, piperidine, morpholine, ethoxylpiperazine, dimethylaminoethylpiperazine or dimethylaminopropylpiperazine; R2 is para-substituted chlorine, fluorine, hydroxyl, methoxyl, dimethylamino, diethylin, methylpiperazine and morpholine, or ortho-substituted hydroxyl and methoxyl or meta-substituted methoxyl and nitro; R4 is hydrogen, chlorine, fluorine, hydroxyl, methoxyl, dimethylamino, diethylin, methylpiperazine or morpholine; R2 and R6 are hydrogen, hydroxyl or methoxyl; R3 and R5 are hydrogen, methoxyl or nitro. Experiments prove that the 2-substituted arylethenyl-N-methylated quinoline derivative provided by the invention has anticholinesterase activity, anti-Abeta aggregation and antioxidant activity.
Owner:SUN YAT SEN UNIV
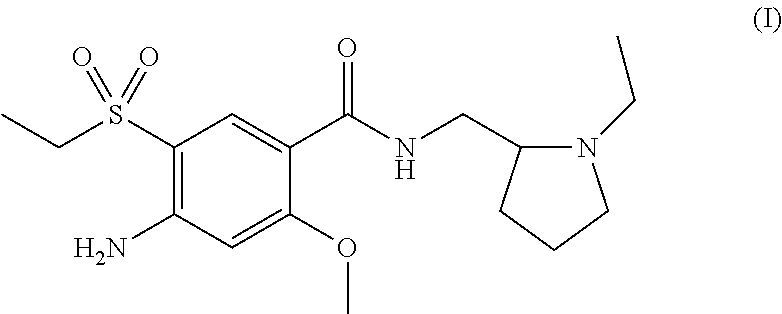
Process for preparation of amisulpride
The present invention is related to a novel process for the preparation of amisulpride (I) which involves: methylation of 4-amino-salicylic-acid (VI) with dimethyl sulphate and base, optionally in presence of TBAB to obtain 4-amino-2-methoxy methyl benzoate (VII) and (ii) oxidation of 4-amino-2-methoxy-5-ethyl thio benzoic acid (IX) or 4-amino-2-methoxy-5-ethyl thio methyl benzoate (X) with oxidizing agent in the presence of sodium tungstate or ammonium molybdate to give 2-methoxy-4-amino-5-ethyl-sulfonyl benzoic acid (IV) or 2-methoxy-4-amino-5-ethyl-sulfonyl methyl benzoate (XI) respectively.
Owner:LUPIN LTD
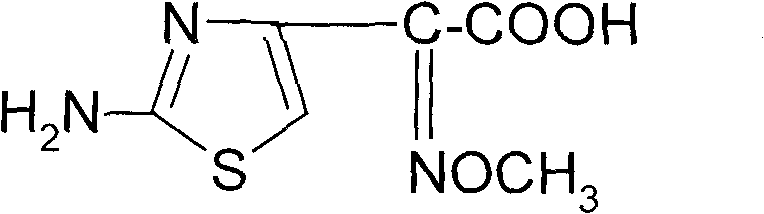
Synthetic method of 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid
ActiveCN101805311AIncrease reaction rateShorten the production cycleOrganic chemistryEthyl acetateHydrolysis
The invention relates to a synthetic method of 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid, which belongs to the synthetic methods of heterocyclic compounds containing 1,3-thiazole ring. The synthetic method is characterized by comprising the following operation steps: 1) homogeneous oximation reaction: preparing 2-hydroxamic ethyl acetoacetate; 2) methylation reaction: preparing 2-methoxyimino ethyl acetoacetate; 3) triphosgene chlorination reaction: preparing 4-chloro-2-methoxyimino ethyl acetoacetate; 4) cyclization reaction: preparing 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid ethyl ester; 5) hydrolysis: preparing a crude product of the 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid; and 6) refining: preparing a product of the 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid. The invention provides an oximating agent system which is applicable to homogeneous nitrosification reaction. The invention provides a triphosgene chlorinating agent which has the advantages of small toxicity, safe and convenient storage, transportation and use, easy control of process operation and high yield. The yield of the 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid ethyl ester is not less than 95.4%; the yield of the crude product of the 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid is not less than 94.4%; and the yield of the finished product of the 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid is not less than 90.5%. The purity of the finished product of the 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid is not less than 99.06%, and the melting point is 182.1 DEG C-183.9 DEG C. The synthetic method is used for synthesizing raw materials of the third generation of cephalosporins.
Owner:YIYUAN XINQUAN CHEM
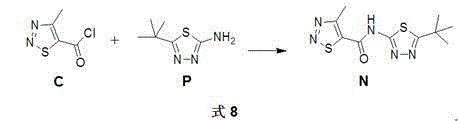
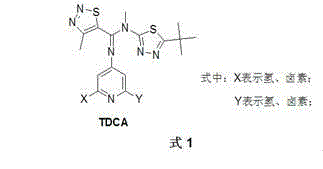
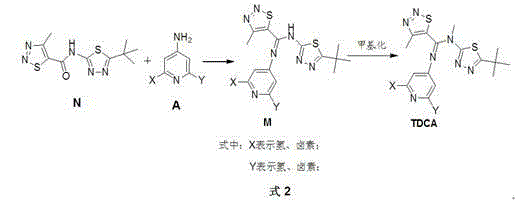
Novel method for synthesizing 1,2,3-thiadiazole-5-formamidine compound
InactiveCN104530040AHigh synthetic yieldSimple ingredientsOrganic chemistryOrganometallic catalysisPtru catalyst
The invention discloses a novel method for synthesizing a 1,2,3-thiadiazole-5-formamidine compound. The target compound shown in general formula TDCA is prepared from a compound as shown in general formula M by virtue of a methylation reaction. The target component as shown in the general formula M is prepared from a compound as shown in general formula A and a compound as shown in general formula N by virtue of a condensation reaction, wherein during the methylation reaction, preferably, a catalyst is an organic metallic catalyst consisting of cuprous iodide and a ligand, namely 2,2,6,6-tetramethyl-3,5-heptadione; during the reaction, preferably, dimethylbenzene is taken as a solvent, and the optimum reaction temperature is 100-140 DEG C. The method disclosed by the invention is high in yield and more environment-friendly (as shown in Specification).
Owner:XIHUA UNIV
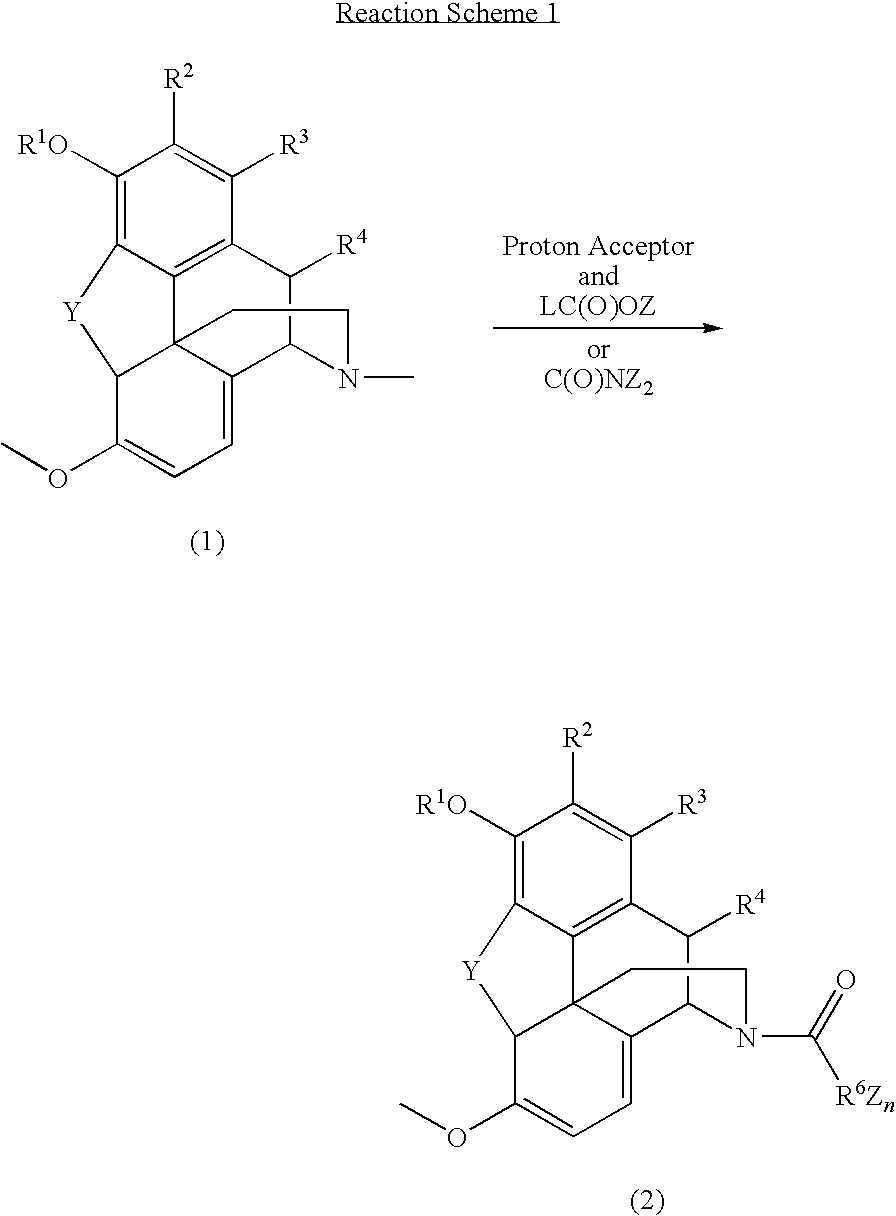
N-demethylation of N-methyl morphinans
The present invention provides a synthetic process for the N-demethylation of N-methyl morphinans. In particular, the invention provides improved synthetic methods for the preparation of N-demethylated morphinan compounds that may be employed as starting materials, for example, commonly available N-methyl opiates such as oripavine and thebaine, and C(3)-protected hydroxy derivatives of oripavine.
Owner:SPECGX LLC
New technology for recycling metribuzin methylate mother liquor
The invention provides a new technology for recycling metribuzin methylated mother liquor. The technology uses bromomethane as methylating agent to react with triazone under alkaline condition and obtain metribuzin; and methylated mother and bromine are recycled for cyclic utilization, thus achieving the aim of the reutilization of wastewater. The inorganic salt content and discharge amount of waste water in methylating working section can be effectively reduced, and the difficulty and cost of treating waste water are largely reduced. The invention has good environmental benefit, social benefit and economic benefit.
Owner:BEIJING ZIGUANG YINGLI CHEM TECH CO LTD
Supplement composition and method of use in enhancement of methylation process
InactiveUS20070021376A1Increase heightIncrease of methylationBiocideSulfur/selenium/tellurium active ingredientsS-Adenosyl-l-methionineMethylsulfonylmethane
A supplement composition for enhancement of methylation process is provided, which contains vitamin B6 (as pyridoxine HCl), folic acid, vitamin B12 (as cyanocobalamin), betaine HCl, and methylsulfonylmethane; and also contains S-adenosylmethionine. The supplement composition further includes silymarin (from milk thistle seed extract), N-acetyl L-cysteine, and cruciferious blend which includes broccoli (brassica oleracea var. talica), kale (brassica oleracea var. acephala), and radish (raphanus sativus). Further provided is a method of using the supplement composition for enhancement of methylation process.
Owner:SURACELL
Novel method for preparing epsilon-caprolactone
ActiveCN106967039AReduce manufacturing costCheap and easy to getOrganic chemistryCyclohexanoneAir atmosphere
The invention discloses a method for preparing epsilon-caprolactone. After cyclohexanone, co-oxidation agents, catalysts, radical initiators and solvents are mixed, reaction is performed in air atmosphere, the co-oxidation agents are methylbenzene compounds, the catalysts are at least one of nitrates or oxides of Co, Fe and Cu, the solvents are at least one selected from 1, 2-dichloroethane, ethyl acetate and acetonitrile, and the method for preparing the caprolactone further co-produces aromatic aldehyde and aromatic acid. The method for preparing the epsilon-caprolactone takes aromatic methyl compounds as the co-oxidation agents, benzaldehyde is replaced, production cost is remarkably reduced, industrial production of the epsilon-caprolactone prepared by an oxygen / air oxidation method is possible, and the aromatic aldehyde and the aromatic acid are further co-produced when the epsilon-caprolactone is prepared by the method, so that the method had higher values in industrial application.
Owner:ZHEJIANG UNIV +1
S-adenosyl-L-methionine analogs with extended activated groups for transfer by methyltransferases
ActiveUS8008007B2Sugar derivativesMicrobiological testing/measurementS-Adenosyl-l-methionineAdenosine
S-Adenosyl-L-methionine analogs of formula (I):are disclosed wherein R comprises a carbon-carbon double bond, carbon-sulfur double bond, carbon-nitrogen double bond, -a carbon-carbon triple bond, carbon-nitrogen triple bond or an aromatic carbocyclic or heterocyclic system in β-position to the sulfonium center, X{circle around (−)} is an organic or inorganic anion carrying one or more negative charges, Z is —CR1R2—, —O—, —S— or —NR3— and R1, R2 and R3 are independently selected from H, D and C1—C alkyl; as well as complexes with a methyltransferase, pharmaceutical compositions, methods for modifying a biomolecule, and methods for detecting sequences specific methylation of biomolecules.
Owner:RWTH AACHEN UNIV +1
Novel synthesizing method of dapoxetine
InactiveCN103304434AHigh yieldHigh stereoselectivityGroup 4/14 element organic compoundsOrganic compound preparationHydroxylaminePtru catalyst
The invention discloses a novel synthesizing method of dapoxetine, wherein a series of reactions are carried out on trans-cinnamaldehyde and N-carbobenzoxy hydroxylamine which serve as initiative raw materials under the action of a self-prepared catalyst (s)-alpha-alpha-diisopropyl dimethyl tert-butyl silicon oxygroup prolinol, so that an important intermediate of the dapoxetine, namely an important chiral intermediate (S)-3-amino-3-phenyl propyl alcohol (compound 4), is obtained. The invention further discloses a preparation method of capecitabine, wherein the compound serving as a raw material is methylated and protected by hydroxyl and then reacts with 1-naphthol to form a salt, so that the capecitabine is obtained. The preparation method is easy in raw material obtainment, good in stereoselectivity and high in yield, thereby being suitable for industrial production.
Owner:湖南欧亚药业有限公司
Dot1l inhibitors for use in the treatment of leukemia
InactiveUS20150342979A1Improve the level ofBiocideMicrobiological testing/measurementDiseaseMedicine
The present invention relates to DOT1L inhibitors. The present invention also relates to pharmaceutical compositions containing these compounds and methods of treating disorders in which DOT1-mediated protein methylation plays a part, such as cancer, by administering these compounds and pharmaceutical compositions to subjects in need thereof.
Owner:EPIZYME
Method for preparing laminine and pharmaceutically acceptable salts thereof
InactiveCN101838213ALess side effectsReduce the cost of separation and purificationOrganic active ingredientsOrganic compound preparationMethylating AgentCarboxyl radical
The invention relates to methods for preparing and using laminine and pharmaceutically acceptable salts thereof. L-lysine is used an initiative material, and the method comprises the following steps of: protecting alpha-amino groups and carboxyl with metallic salts under an alkaline condition to form a chelate complex; performing separation and purification to obtain the chelate complex with the purity of 99 to 100 percent, and performing methylation on the chelate complex on epsilon-amino groups under the action of a phase transfer catalyst with a methylating agent to generate trimethyl lysine salts; removing metallic ions with a precipitator or chelating agent to obtain crude laminine and crude pharmaceutically acceptable salts thereof; and re-crystallizing the crude laminine and the crude pharmaceutically acceptable salts thereof with a solvent to obtain the medicinal laminine and the pharmaceutically acceptable salts thereof. The methylation of the separated and purified L-lysine metallic ion chelate complex and the phase transfer catalyst are simultaneously adopted, so the method remarkably reduces the generation of monomethyl substances, dimethyl substances and other byproducts, improves the yield and achieves the product purity of over 98.5 percent (HPLC).
Owner:QINGDAO UNIV OF SCI & TECH
Hydroaminomethylation of olefins
ActiveUS20050215825A1Organic compound preparationCarboxylic acid amides preparationSyngasFexofenadine
The present invention relates to a method comprising the step of contacting under hydroaminomethylation conditions, an olefin, an amine, a rhodium-phosphorous ligand, and synthesis gas (syngas). In particular, it has been discovered that, under some circumstances, a neutral rhodium-monodentate phosphite ligand is prescribed. The invention provides a simple way of making, in high yields and regiospecificity, a variety of products, including pharmacologically active products such as ibutilide, terfenadine, and fexofenadine, and derivatives thereof.
Owner:UNION CARBIDE CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com