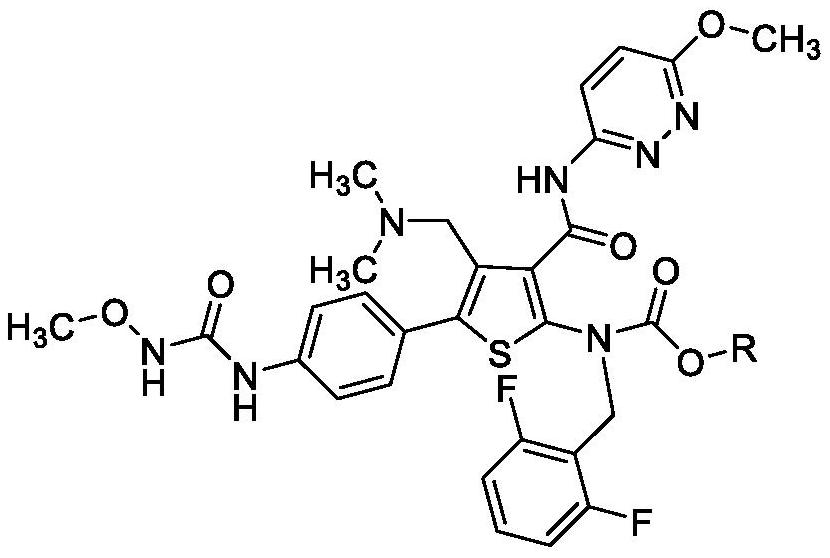
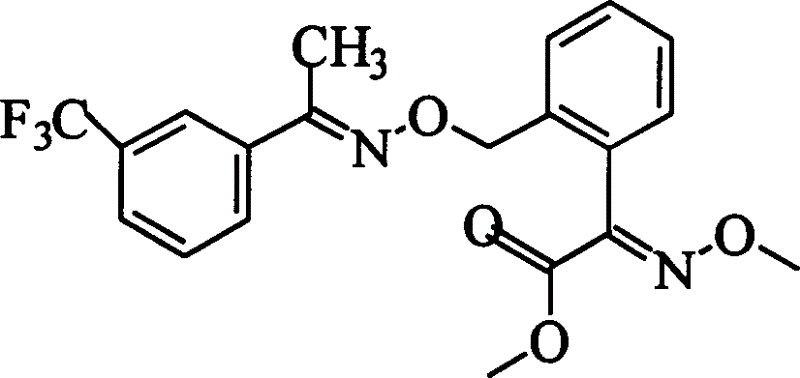
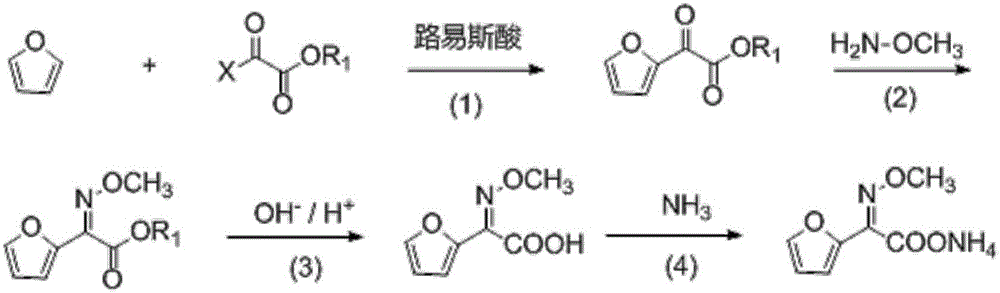
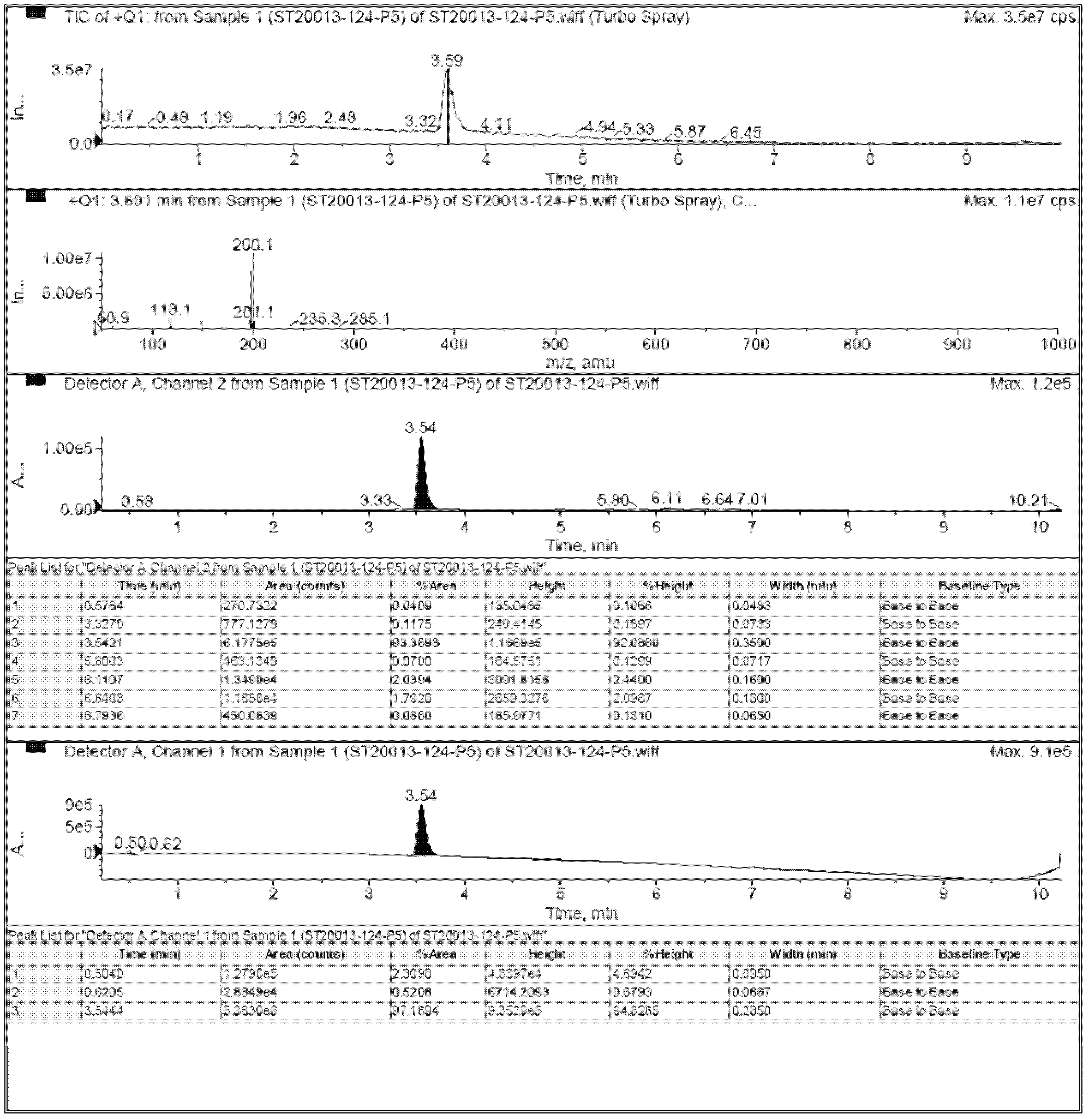
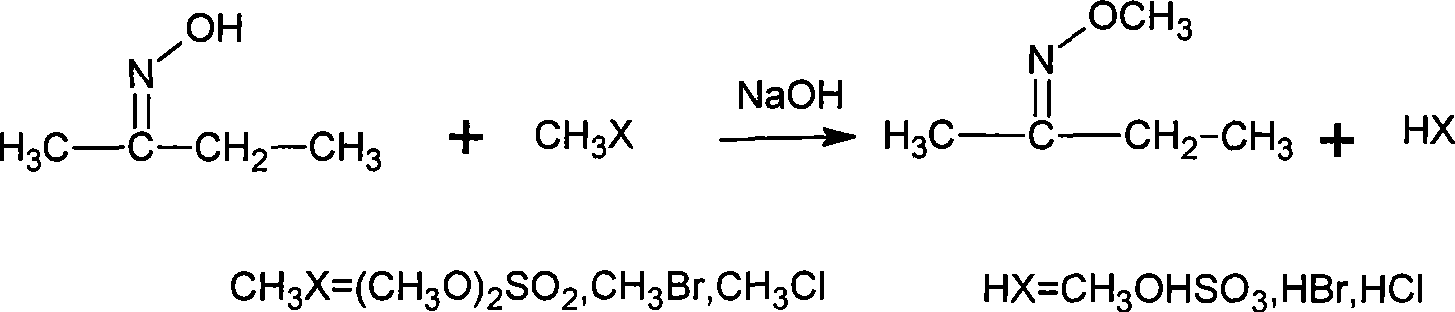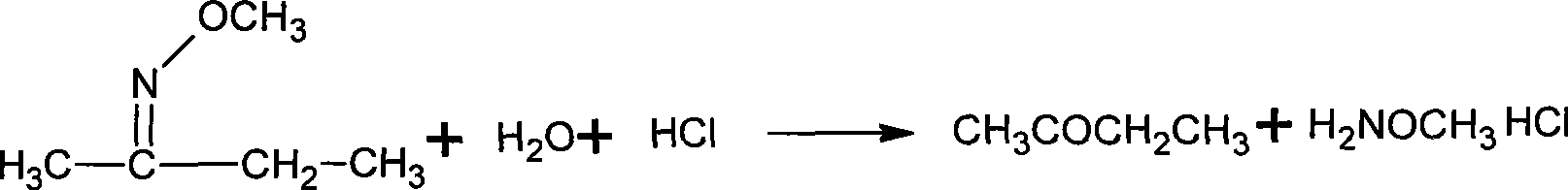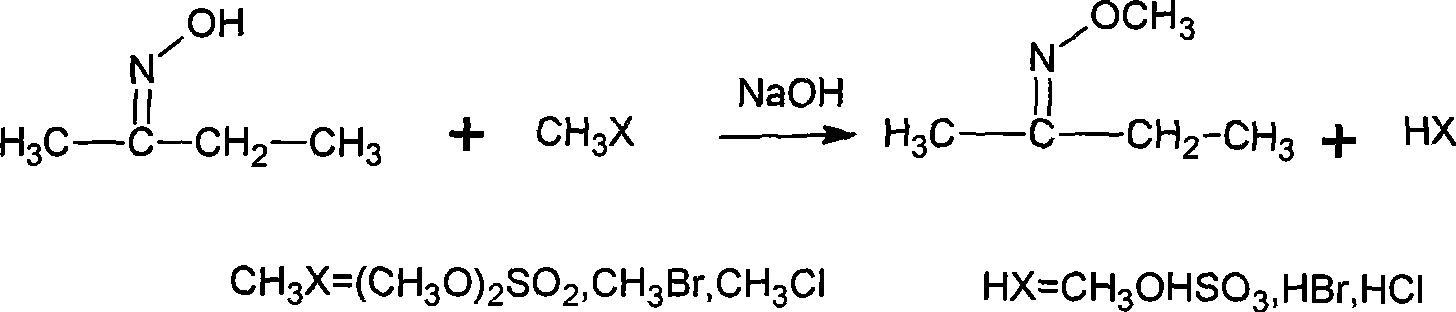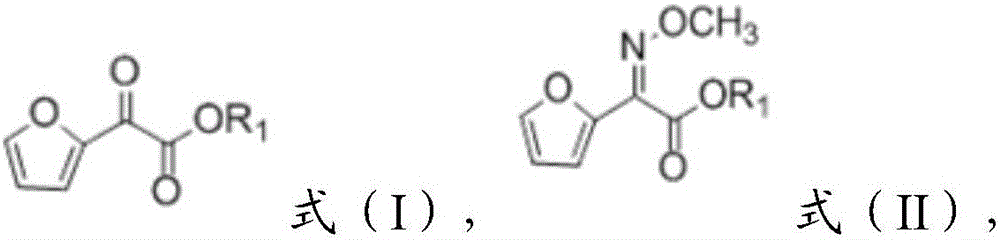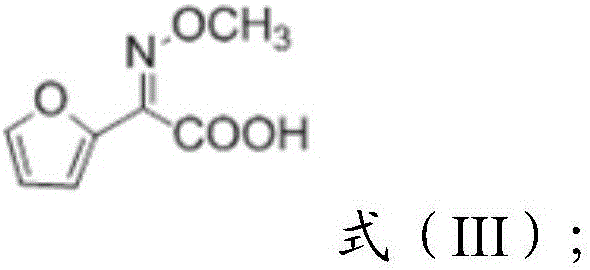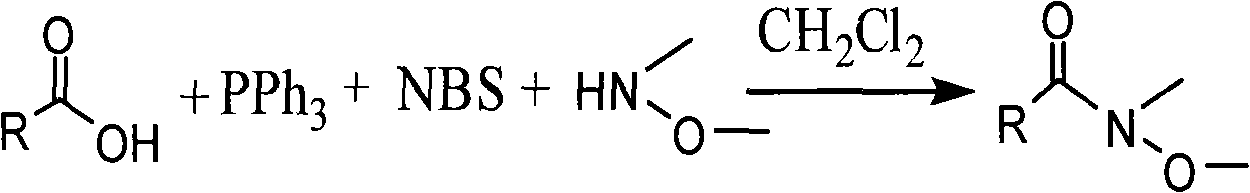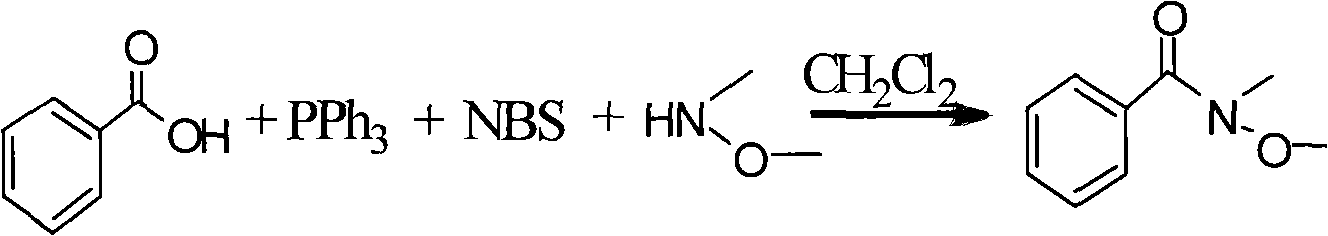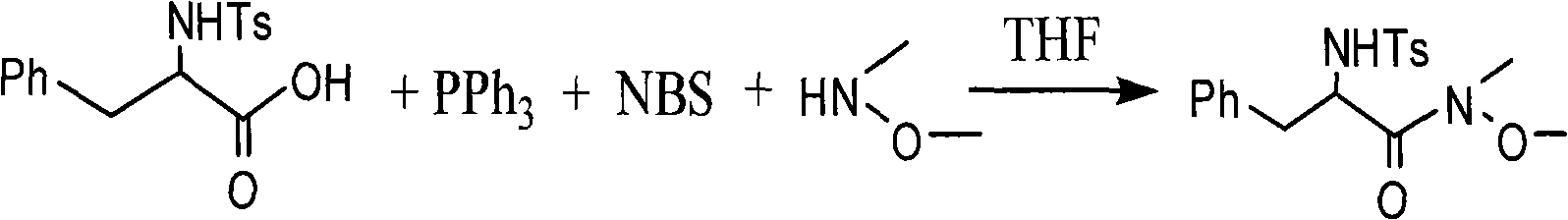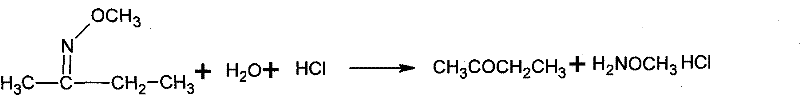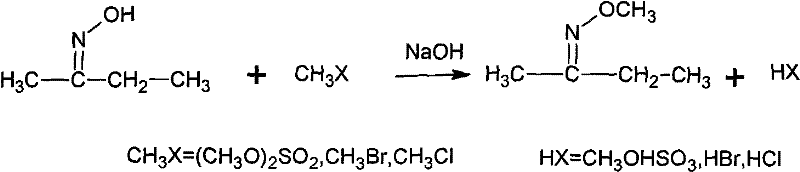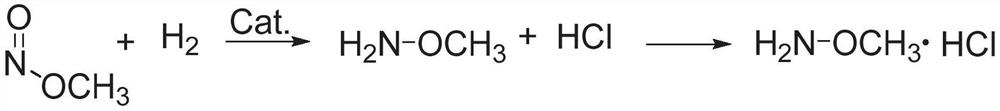Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Methoxyamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methoxyamine is the organic compound with the formula CH₃ONH₂. Also called O-methylhydroxylamine, it a colourless volatile liquid that is soluble in polar organic solvents and in water. It is a derivative of hydroxylamine with the hydroxyl hydrogen replaced by a methyl group. Alternatively, it can be viewed as a derivative of methanol with the hydroxyl hydrogen replaced by an amino group. It is an isomer of N-methylhydroxylamine and aminomethanol. It decomposes in an exothermic reaction (-56 kJ/mol) to methane and azanone unless stored as a hydrochloride salt.
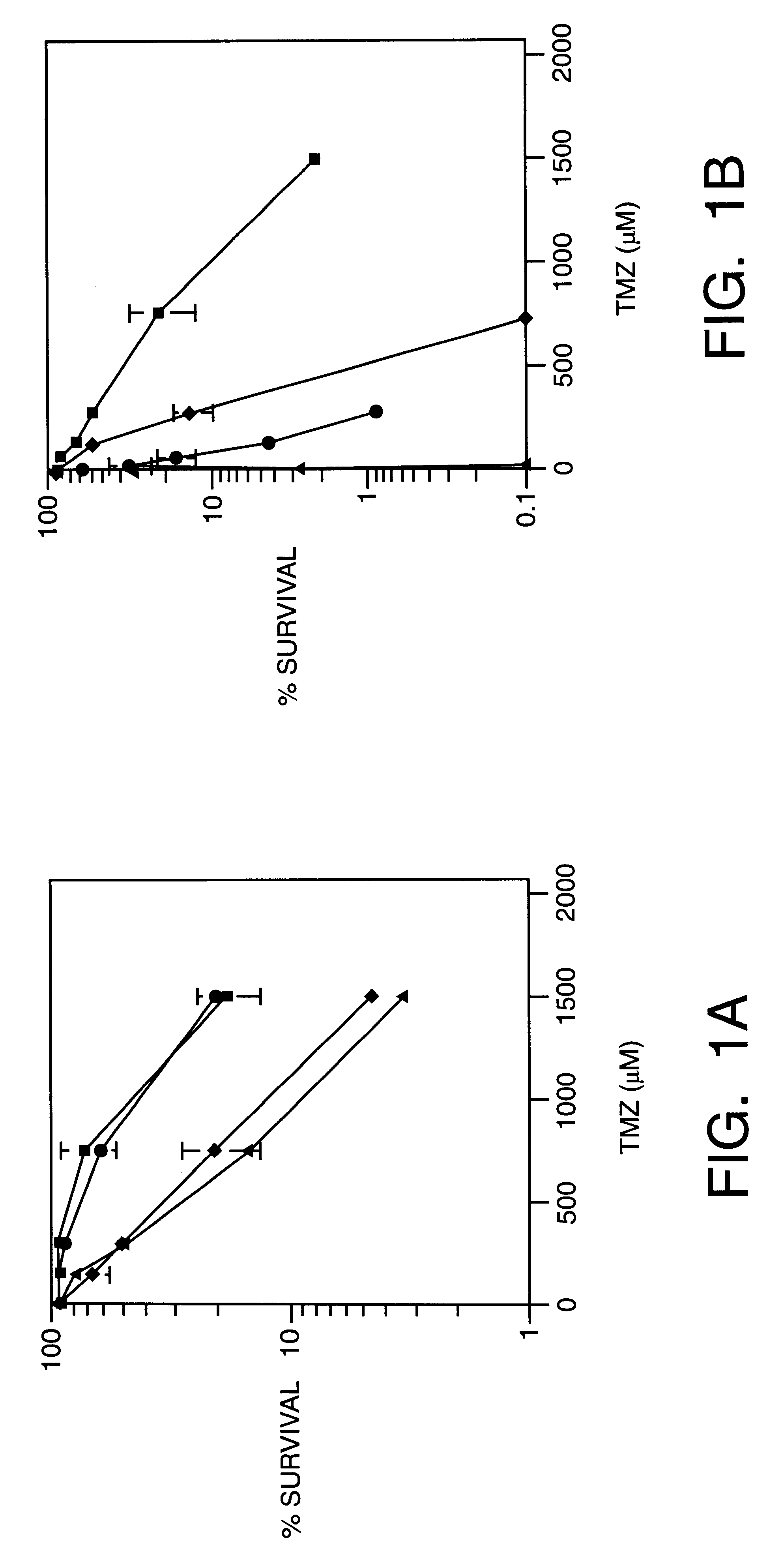
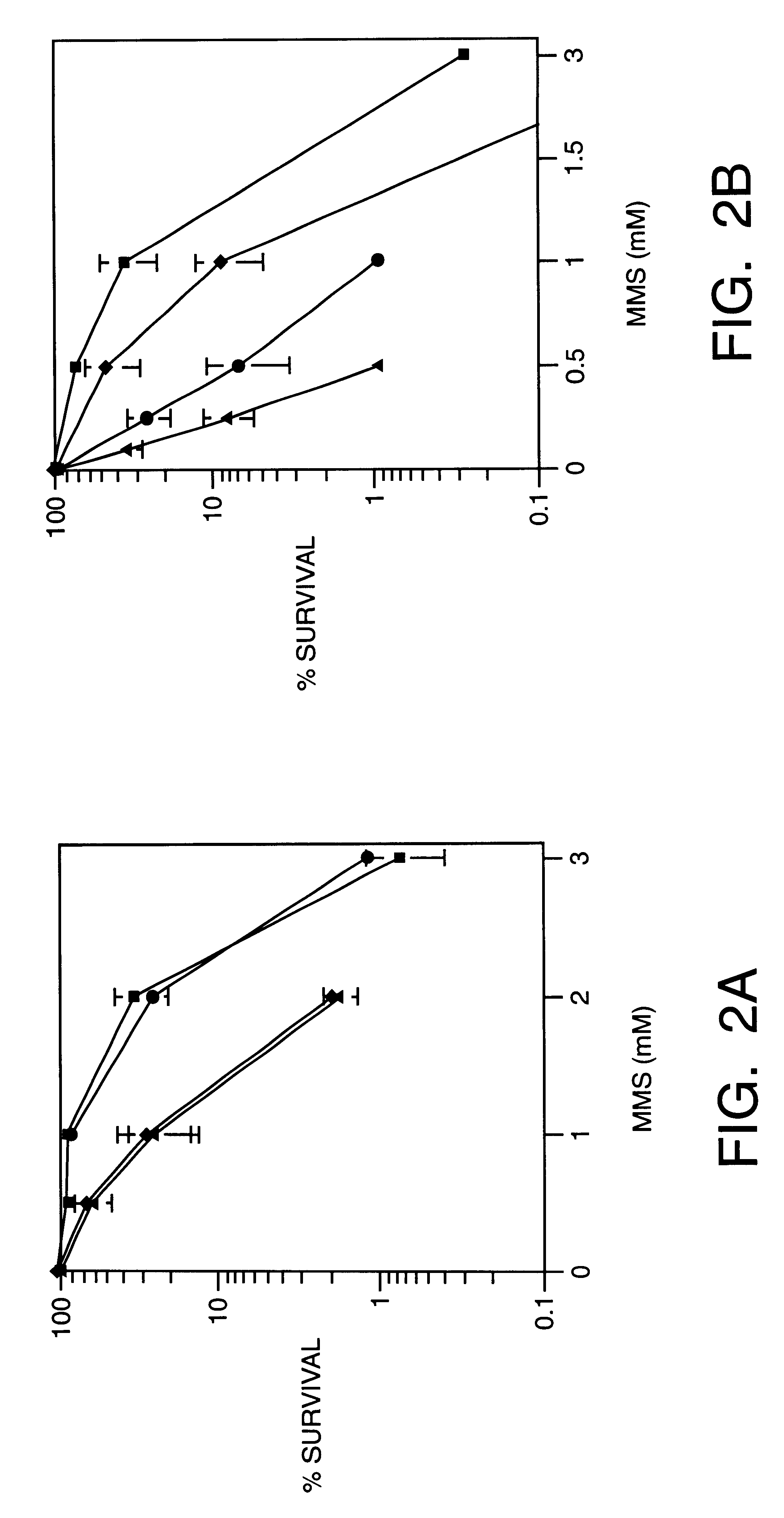
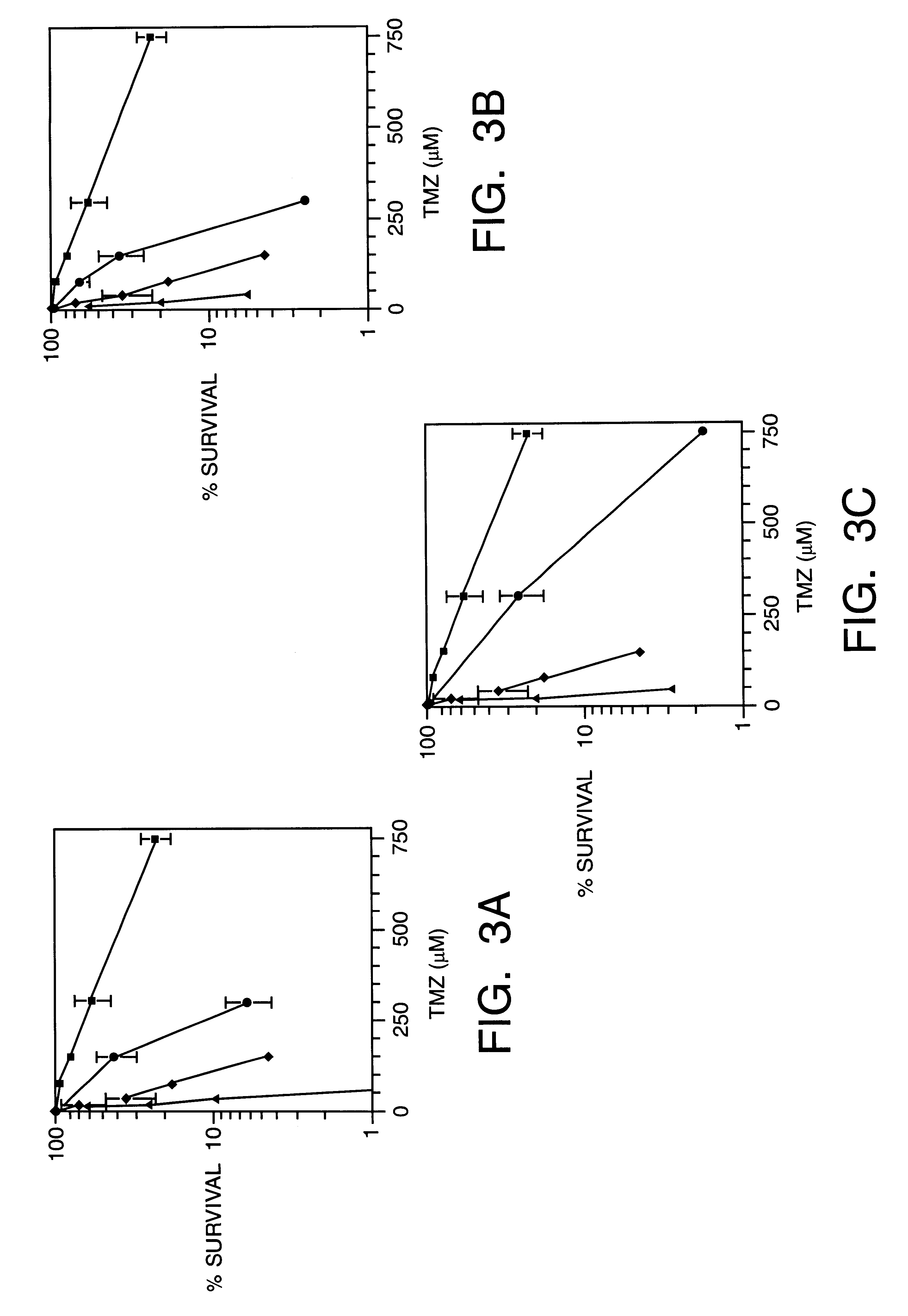
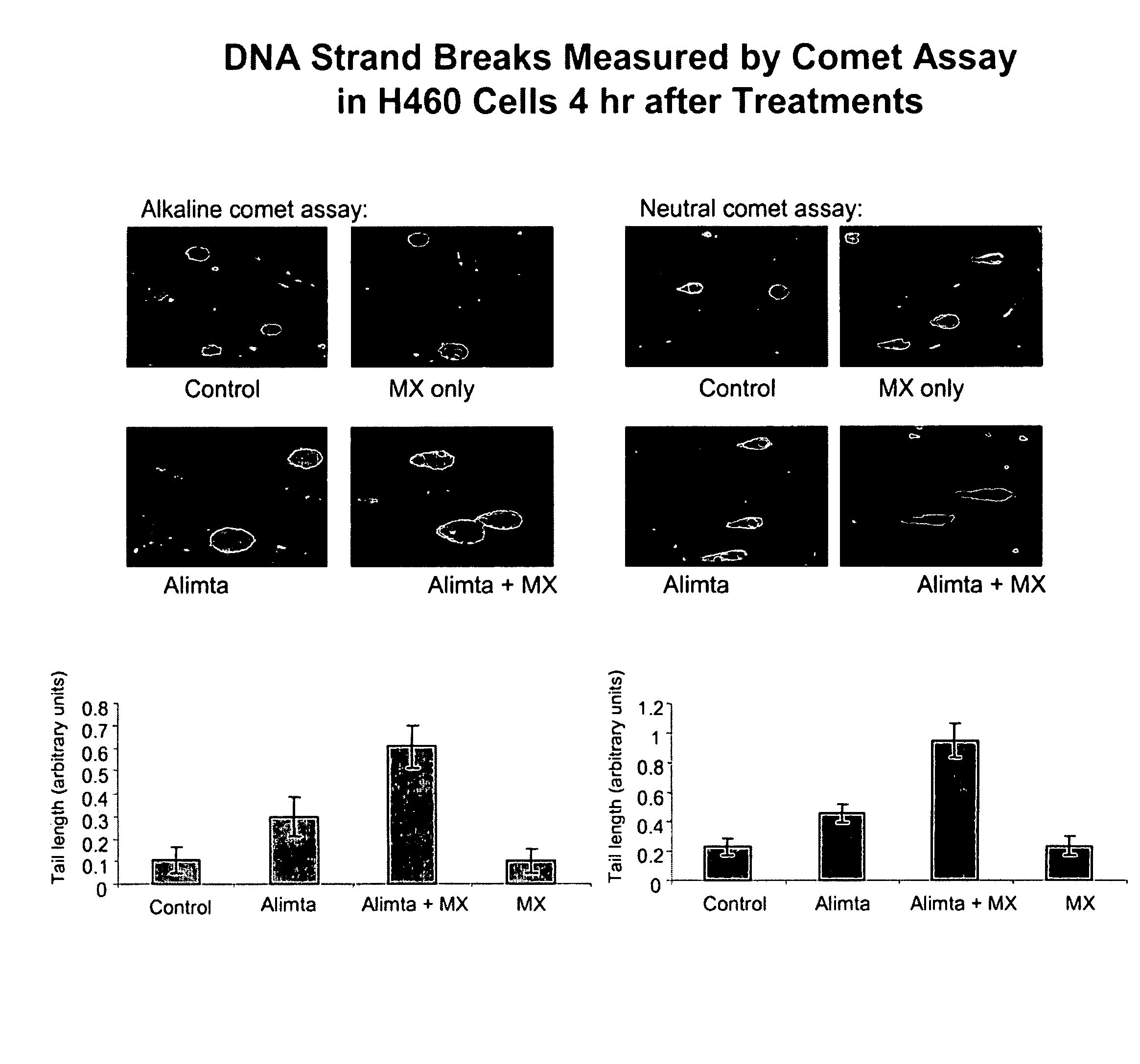
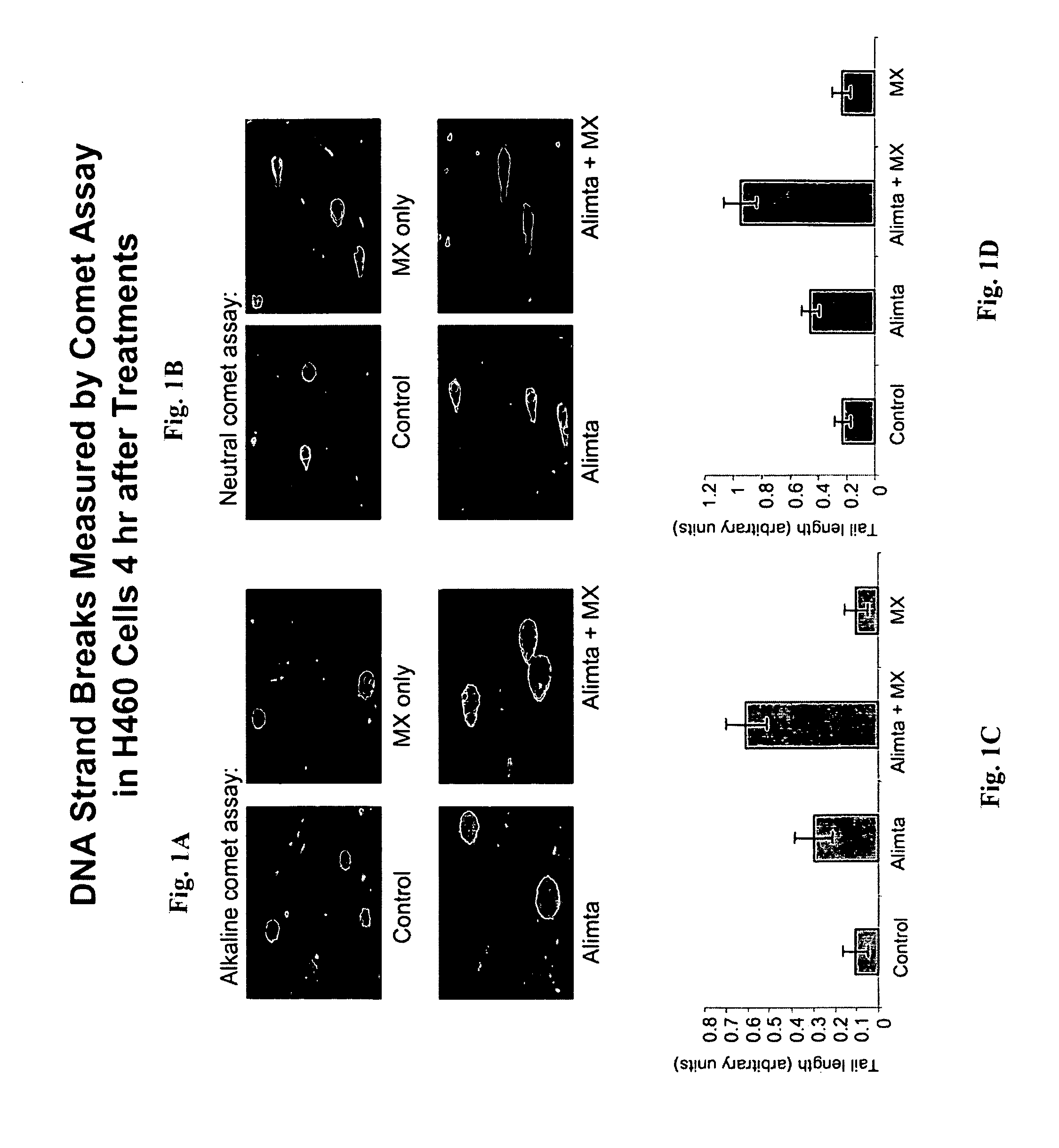
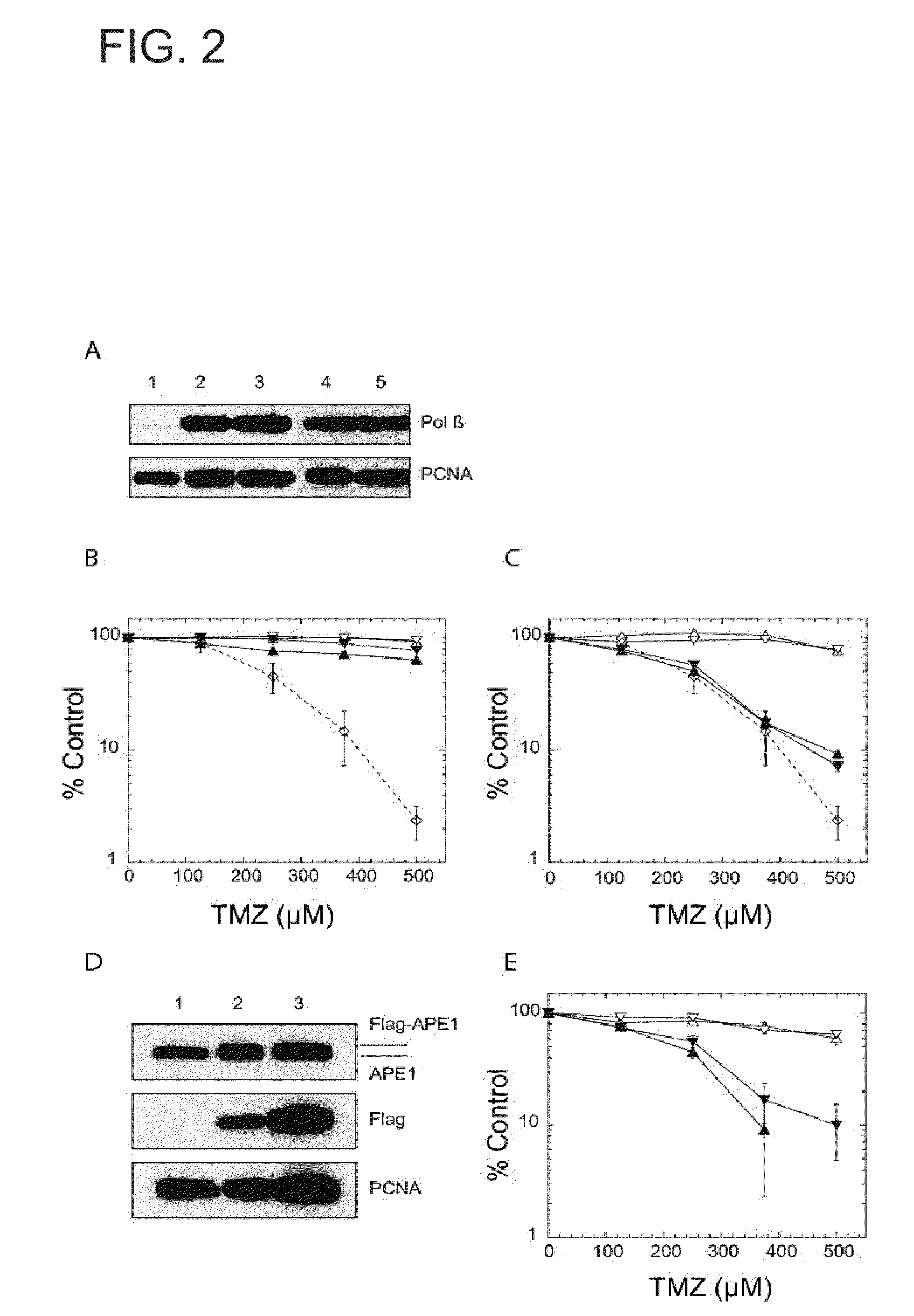
Methoxyamine potentiation of temozolomide anti-cancer activity
InactiveUS6465448B1Prevents APE cleavageDisrupting DNA repairBiocideAnimal repellantsMethoxyamineTemozolomida
This invention generally relates to novel compositions and methods for the treatment of certain cancers. Additionally, this invention relates to novel compositions and methods to screen drugs for the treatment of certain cancers. Specifically, the invention contemplates that temozolomide and methoxyamine, in combination or in sequence, shall be used as a treatment for certain tumors that are resistant to treatment by temozolomide alone.
Owner:CASE WESTERN RESERVE UNIV
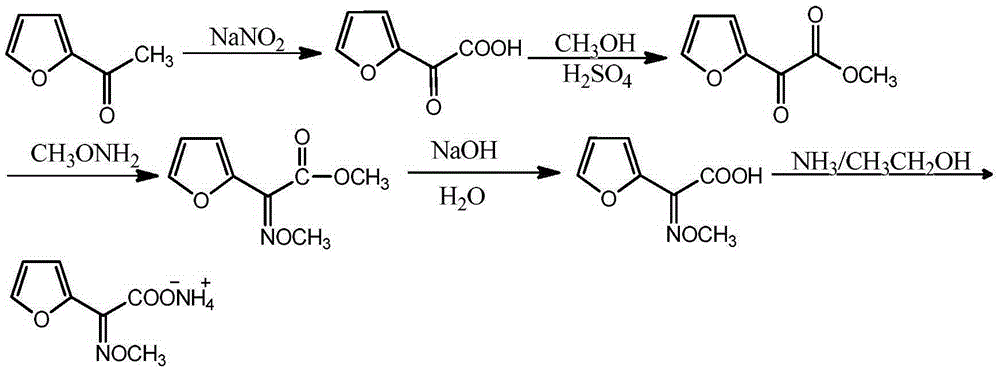
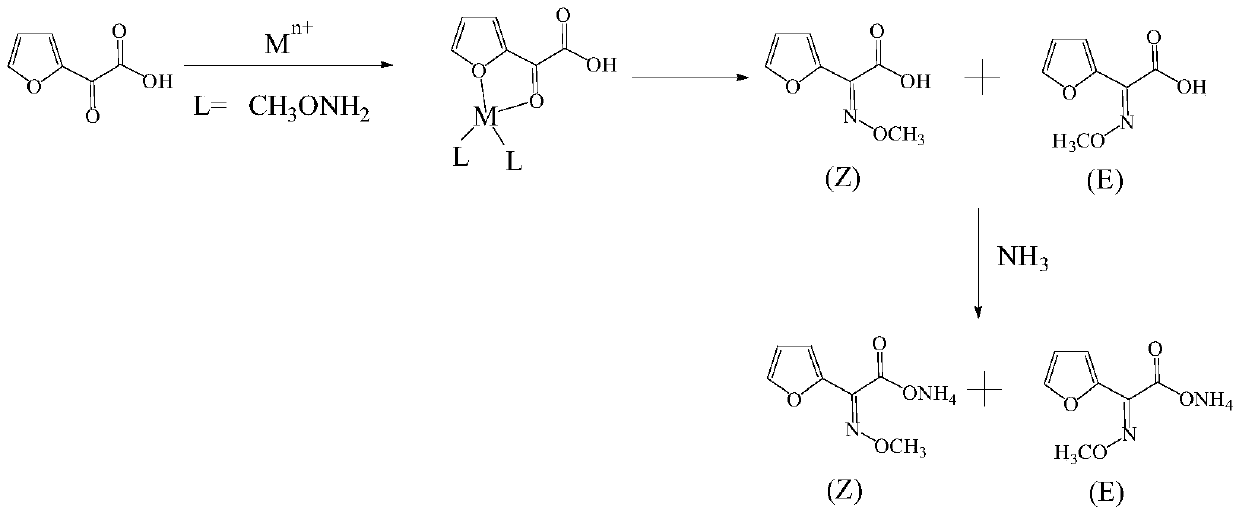
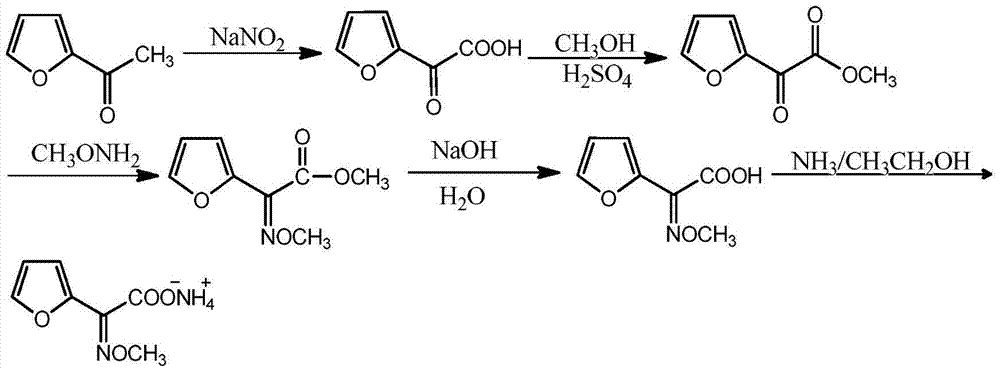
Synthetic technology of furan ammonium salt
The invention belongs to the technical field of medical intermediate preparation, and particularly relates to a synthetic technology of furan ammonium salt. The synthetic technology comprises the steps that 2-acetyl furan is used as a raw material to form furanone acid through oxidative synthesis at first, then esterification is performed, the product reacts with methoxyamine to be synthesized into a methoxy group oximation product, the methoxy group oximation product is hydrolyzed and then reacts with an alcohol amine solvent, and the furan ammonium salt is obtained. Due to the fact that the furanone acid is esterified at first, the carbonyl activity is improved, and more oximation products are promoted to be converted into cis-form products. The content of the obtained anti-form furan ammonium salt is only 5-8%; compared with a traditional furan ammonium salt production method, the content of the anti-form furan ammonium salt is obviously reduced without lowering productivity.
Owner:SHANDONG JINCHENG PHARMACCUTICAL CHEM CO LTD
Method for synthesizing methoxy amine hydrochlorate
InactiveCN101503375AEmission reductionMild reaction conditionsOrganic chemistryEvaporationOrganic layer
The invention discloses a synthesizing method of methoxy amine hydrochloride. Butanone oxime, water, sodium hydroxide, phase transfer catalyst are added in a reaction container; the temperature is decreased to 0-15 DEG C; a methylating reagent begins to be added; then, reaction is carried out at a temperature of 5-120 DEG C for 3-6 h; standing and layering are carried out so that an organic layer O-methyl-2-diacetylmonoxime ether and a water layer are separated; then, the O-methyl-2-diacetylmonoxime ether in the water layer is distilled and recovered under the normal pressure; the separated O-methyl-2-diacetylmonoxime ether is mixed with the distilled and recovered O-methyl-2-diacetylmonoxime ether and then with hydrochloric acid solution with a mass percentage of 30-35 percent; the mixed solution undergoes feeding and rectification at the middle of a rectifying column; butanone and methanol are recovered by the column top; methoxy amine hydrochloride solution is recovered by the column bottom; and the methoxy amine hydrochloride solution also undergo evaporation and dehydration by heating so as to obtain a hydrochloride concentrated solution thereof. The synthesizing method is simple, generates a small amount of three wastes and improves the operation environment and the yield rate.
Owner:宁波四明化工有限公司 +1
Preparation method for methoxylamine hydrochloride
InactiveCN102976968AAvoid toxic substancesEmission reductionOrganic chemistryHydroxylamineNitrogen oxides
The invention discloses a preparation method for methoxylamine hydrochloride, comprising the following preparation steps of: 1) preparing acetyl hydroxylamine; 2) preparing acetyl methoxyamine; and 3) preparing methoxylamine hydrochloride. The preparation method is moderate in reaction conditions, and is capable of avoiding the toxic substances such as sulfur dioxide and sodium nitrite which are used in the existing production process for methoxylamine hydrochloride, reducing the emission of waste gases such as nitric oxide, and lightening a pressure of environment.
Owner:张家港市大伟助剂有限公司
Method for producing furan ammonium salt by using furoic acid
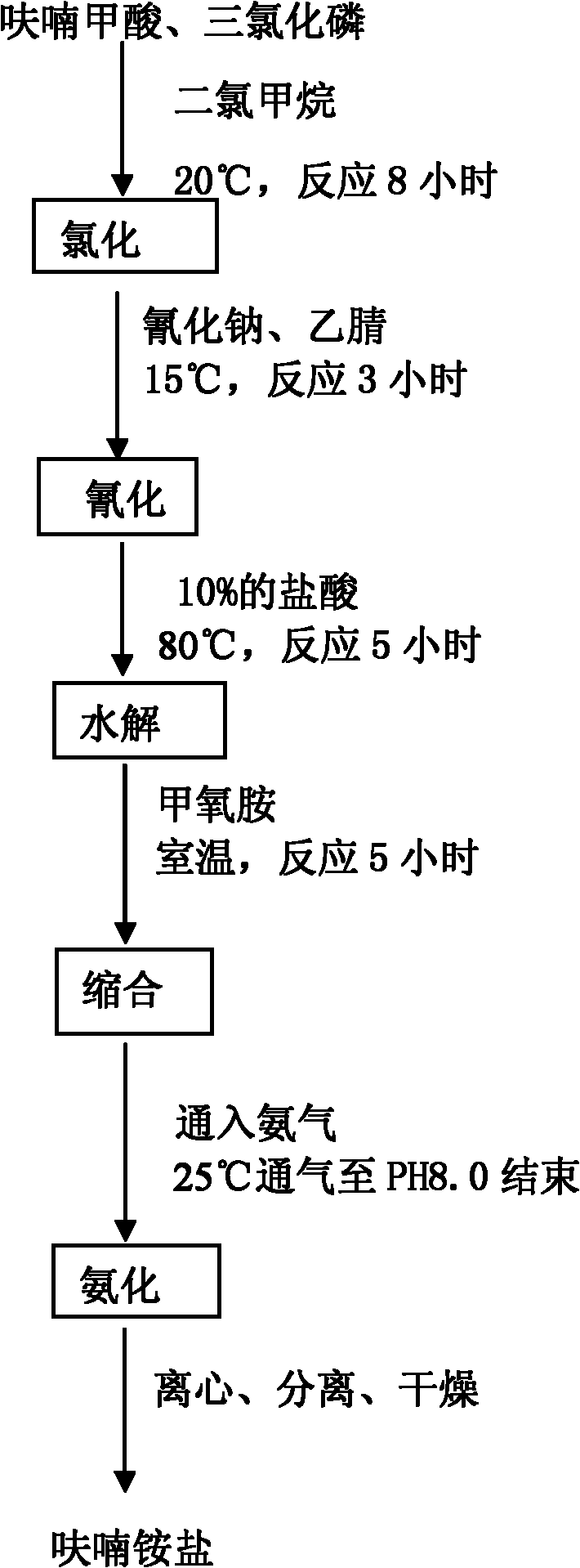
The invention discloses a method for producing a furan ammonium salt by using furoic acid. The method comprises the following steps of: (1) chlorination: putting furoic acid and chlorohydrocarbon solvent into an enameled glass reaction kettle, controlling temperature and time, and obtaining a furoyl chloride product; (2) cyanation: putting the furoyl chloride and sodium cyanide into the reaction kettle, stirring, preserving heat, reducing pressure, and reclaiming the solvent to obtain a furoyl nitrile product; (3) hydrolysis: heating the furoyl nitrile and hydrochloric acid, and obtaining furan ketone acid after the reaction is finished; (4) condensation: adding ethyl acetate extract of the furan ketone acid serving as the hydrolysis product into the reaction kettle, dropping methoxyamine to perform room temperature reaction with the hydrolysis product, and demixing, wherein the oil layer is used for ammoniation reaction; and (5) ammoniation: introducing ammonia gas into the oil layer reactant, controlling the temperature, stirring, finishing gas introduction, centrifuging, and drying to obtain the furan ammonium salt. The method is simple and convenient, is convenient to operate, reduces the production cost, improves the yield, makes full use of raw materials, and is safe and reliable in the production process.
Owner:湖北楚阳科技股份有限公司
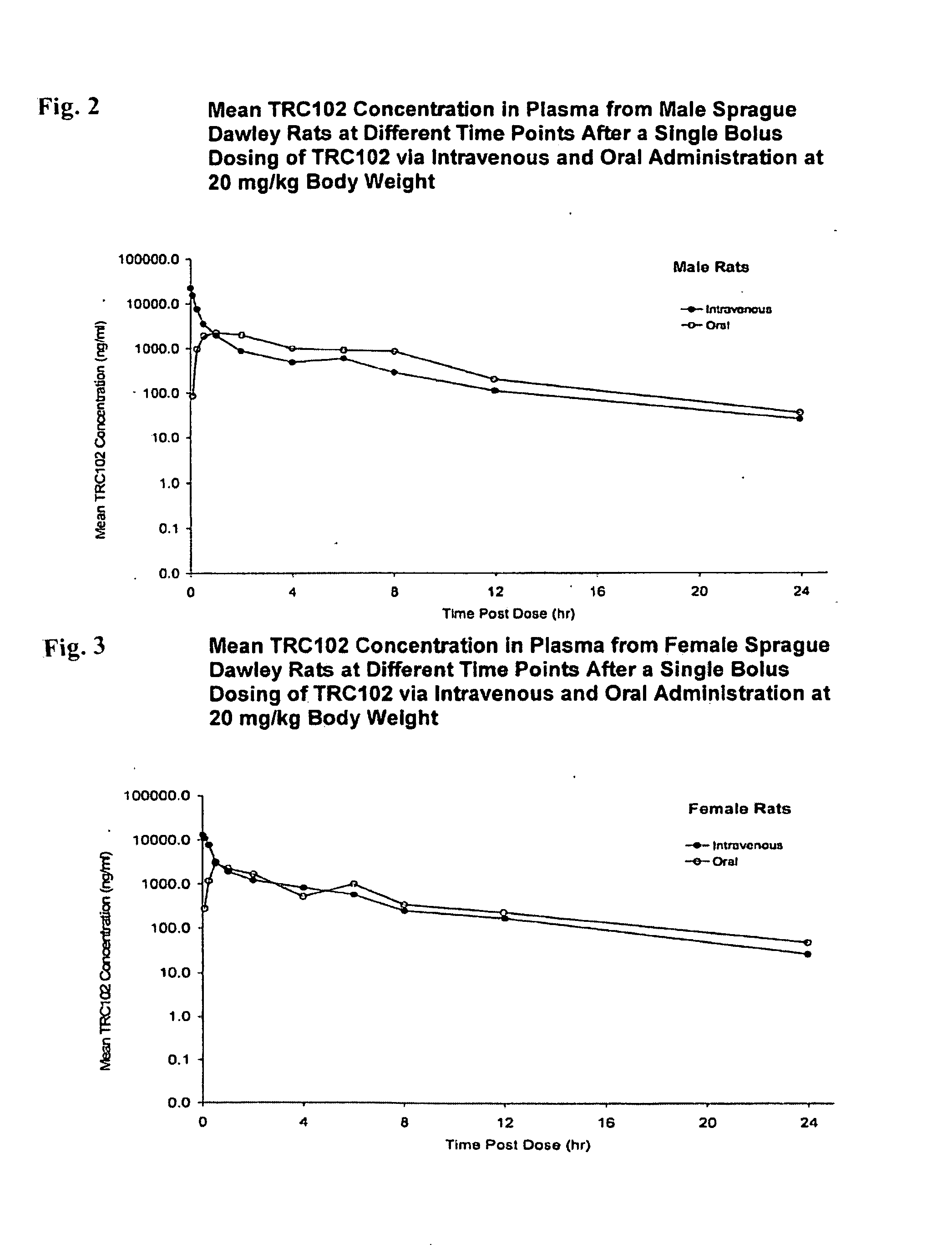
Antifolate agent combinations in the treatment of cancer
ActiveUS20080234298A1Enhance and increase effectSufficient amountBiocideAmine active ingredientsAP siteMethoxyamine
Compositions and methods useful in the treatment of certain cancers. The methods include administering, to a patient receiving an antifolate anticancer agent, methoxyamine administered in an amount sufficient to enhance or increase the effect of the antifolate anticancer agent. In part, this application is based on the recognition that certain molecules that target abasic lesions or AP sites in DNA improve, augment, or potentiate the chemotherapeutic efficacy of certain anticancer agents.
Owner:TRACON PHARMA
Preparation method of furan ammonium salt
The invention discloses a preparation method of a furan ammonium salt. The preparation method of the furan ammonium salt comprises the following steps: taking 2-furyl-methylketon as the raw material,enabling 2-furyl-methylketon and sodium nitrite to react under the catalytic action of a metal salt to obtain a furan keto ester; enabling the furan keto ester and methoxyamine to react to obtain 2-methoxy-imine-2-furan methyl acetate; regulating the pH value of a 2-methoxy-imine-2-furan methyl acetate solution, extracting with an organic solvent to obtain a 2-methoxy-imine-2-furan acetic acid solution; and enabling the 2-methoxy-imine-2-furan acetic acid solution and an alcohol amine solvent to react to obtain the furan ammonium salt. The metal salt in the step S1 is one or more of sulfates of a ferric salt, a copper salt and a zinc salt. On the one hand, the yield of the furan ammonium salt is increased by improving the conversion rate of furyl methylketon, and on the other hand, the yield of the furan ammonium salt is increased by reducing the content of trans-furan ammonium salt.
Owner:CHENGDU ORGANOCHEM CO LTD
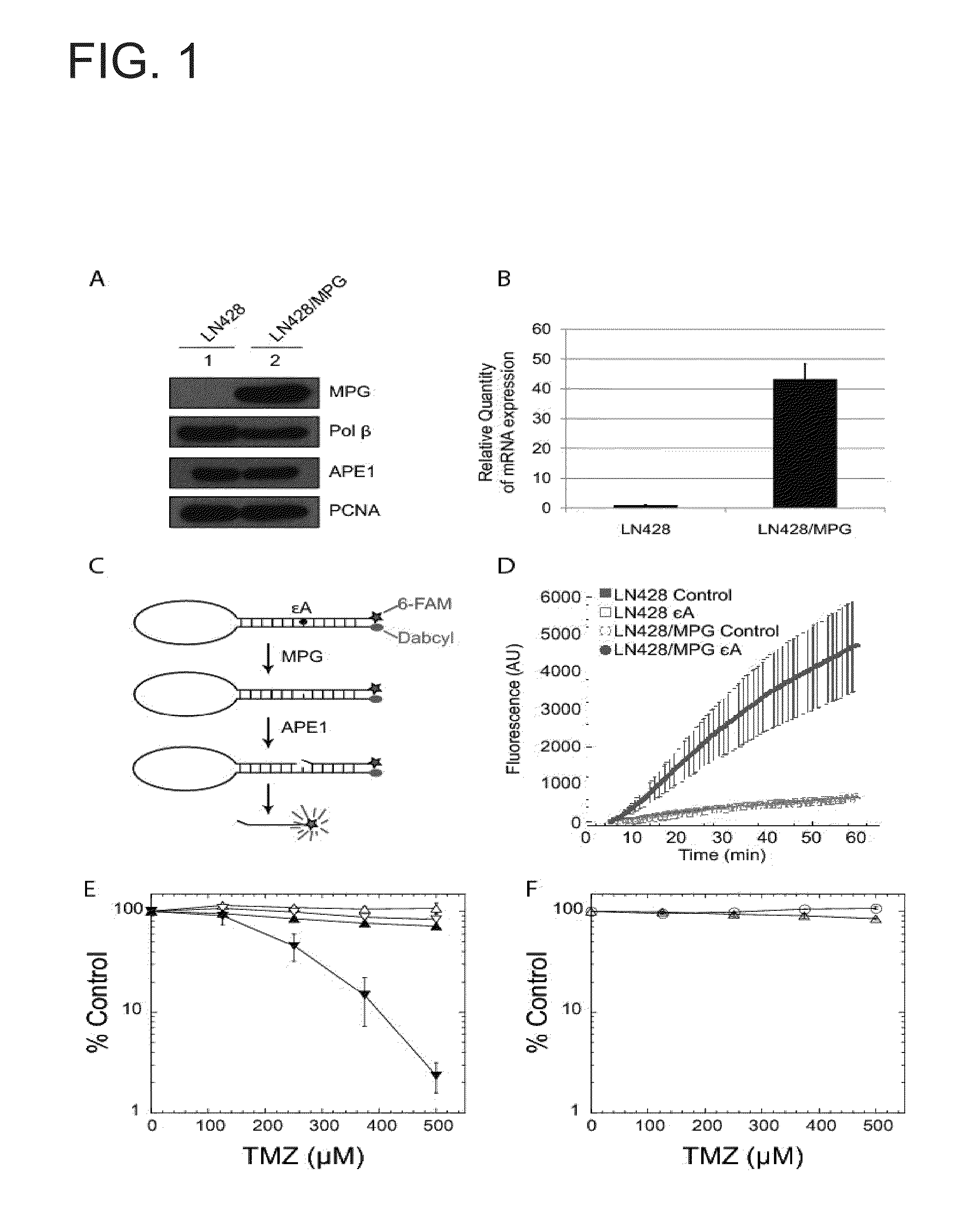
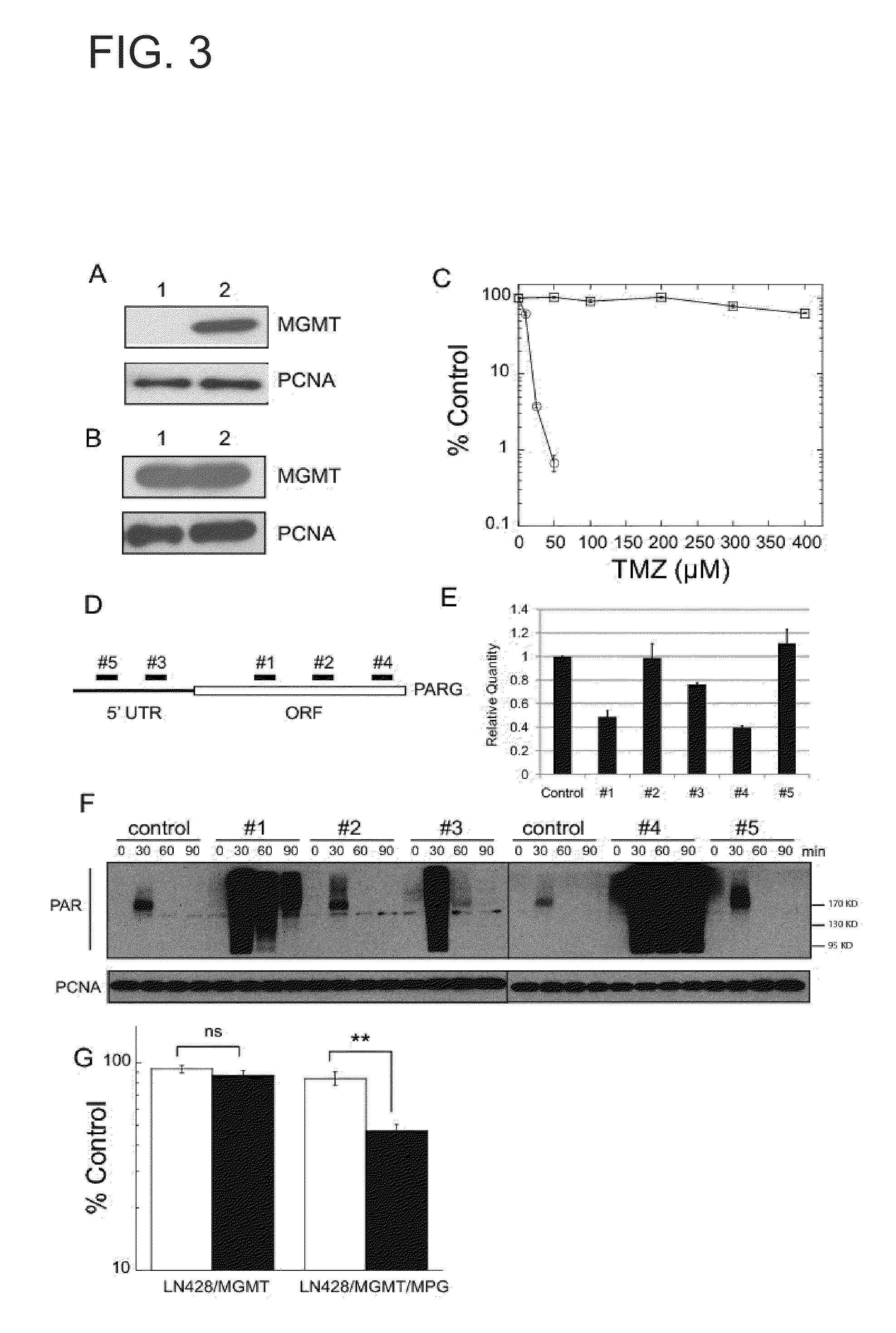
N-methylpurine DNA glycosylase and polymerase beta as biomarkers for alkylator chemotherapy potentiation
InactiveUS20110245309A1Reduce expressionHigh expressionOrganic active ingredientsBiocideMethylpurine DNA GlycosylaseN-METHYLPURINE DNA GLYCOSYLASE
Described herein is the finding that polymerase β (Polβ) and N-methylpurine DNA glycosylase (MPG) can be used as biomarkers to evaluate the sensitivity of a subject to combination therapy that includes treatment with either temozolomide (TMZ) and methoxyamine, or TMZ and a poly(ADP-ribose) polymerase (PARP) inhibitor. Thus, provided herein is a method of determining if a subject will be sensitive to TMZ and methoxyamine, or TMZ and a PARP inhibitor by measuring expression of Polβ and MPG in a sample from the subject and comparing expression of Polβ and MPG in the sample to a control. A decrease in expression of Polβ and an increase in expression of MPG relative to the control indicates the subject is sensitive to TMZ and methoxyamine, or sensitive to TMZ and the PARP inhibitor.
Owner:UNIVERSITY OF PITTSBURGH
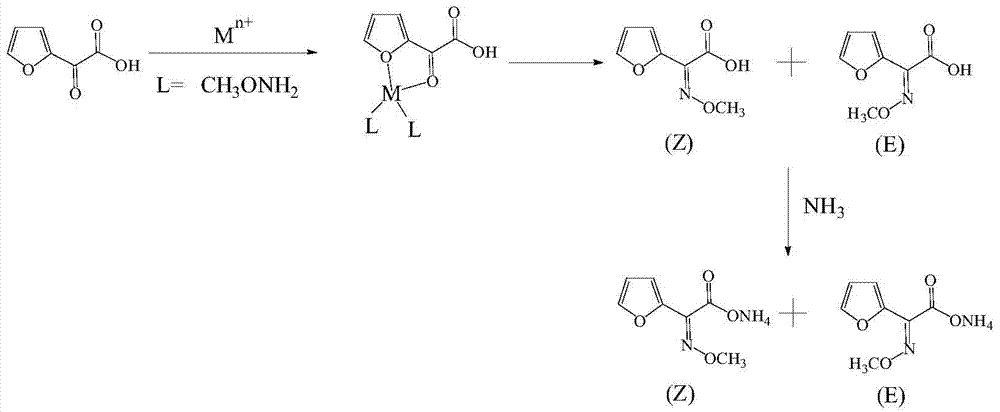
Method for synthesizing (Z)-2-(alpha-methoxyimino)furanylacetic acid ammonium
The invention belongs to the technical field of medical intermediate preparation, and particularly relates to a method for synthesizing (Z)-2-(alpha-methoxyimino)furanylacetic acid ammonium. The method comprises the following steps that 1, 2-oxo-2-furanylacetic acid and heavy metal salt are dissolved in water, an aqueous methoxyamine solution or aqueous methoxyamine salt solution is added at the temperature of 0 DEG C-10 DEG C, and the pH is regulated to 2.5-3.5; 2, heat preservation is performed for 2-7 h at the temperature of 5 DEG C-10 DEG C, and a 2-(alpha-methoxyimino)furanylacetic acid solution is obtained; 3, the pH of the 2-(alpha-methoxyimino)furanylacetic acid solution is regulated to 0.1-1.5 by adopting inorganic acid, the temperature is controlled at 15 DEG C-25 DEG C, extraction is performed through organic solvent, and organic phases are combined; 4, ammonia gas or liquid ammonia is introduced into the organic phases at the temperature of 0 DEG C-10 DEG C, the pH is regulated to 6.5-7.5, heat preservation is performed for 0.5-1.5 h to obtain a crude product, and after decoloration, concentration and crystallization are performed, the product is obtained. The method is high in yield and good in product quality.
Owner:SHANDONG JINCHENG PHARMACCUTICAL CHEM CO LTD
Method for preparing furan ammonium salt with high selectivity
The invention belongs to the technical field of preparation of medical intermediates, particularly relates to a method for preparing furan ammonium salt with high selectivity, and solves the problems of low conversion rate of a raw material 2-acetylfuran, low yield of furan ammonium salt, harsh reaction conditions of a furan ammonium salt preparation process and the like in the prior art. The method comprises the following steps: adding water, an acidic solution and 2-acetylfuran into an oxidation kettle, heating, dropwise adding an oxidant, and reacting to obtain 2-oxo-2-furanacetic acid; cooling 2-oxo-2-furanacetic acid, and then adding methoxyamine to react, so as to obtain 2-methoxyamine-2-furanacetic acid; adding methanol an ammoniation salifying kettle, adding 2-methoxyamine-2-furanacetic acid, introducing ammonia gas for a reaction, and obtaining the furan ammonium salt. The synthesis raw materials are low in price and easy to obtain, the preparation process is mild in reaction condition, safe and environmentally friendly, the yield and purity of the furan ammonium salt product are improved, and the production cost is reduced.
Owner:安徽金轩科技有限公司
Preparation method and application of 2-(4-beta-D-allose pyranoside-phenyl)-2,3-dihydroquinoline-4(1H) and 2-(4-(2,3,4,6-tetrabenzyl)-beta-D-allose pyranoside-phenyl)-2,3-benzodihydropyran
The invention relates to a preparation method and an application of 2-(4-beta-D-allose pyranoside-phenyl)-2,3-dihydroquinoline-4(1H) (1) and 2-(4-(2,3,4,6-tetrabenzyl)-beta-D-allose pyranoside-phenyl)-2,3-benzodihydropyran (2). The invention discloses helicide derivatives with a central nervous system improving function, and a preparation method thereof. The compounds provided by the invention have good sedative, sleep-promoting, and convulsion-inhibiting functions. The compounds are collected in a Year 2000 version of Pharmacopoeia of PRC with a medicine name of helicide tablets. The helicide derivative novel medicines are represented by chemical formulas (1) and (2), wherein R1 can be hydrogen and acetyl, and R2 can be semicarbazide, hydroxylamine, and methoxylamine. Acording to the helicide derivatives provided by the invention, a plant active monomer helicide with sedative, sleep-promoting, and convulsion-inhibiting functions is adopted as a matrix compound, structural modification is carried out according to a medicine efficacy molecular principle, and compounds with high activities for improving central nervous system functions are designed and prepared.
Owner:SICHUAN UNIV
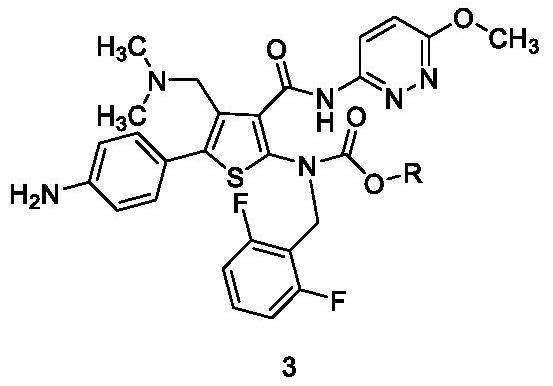
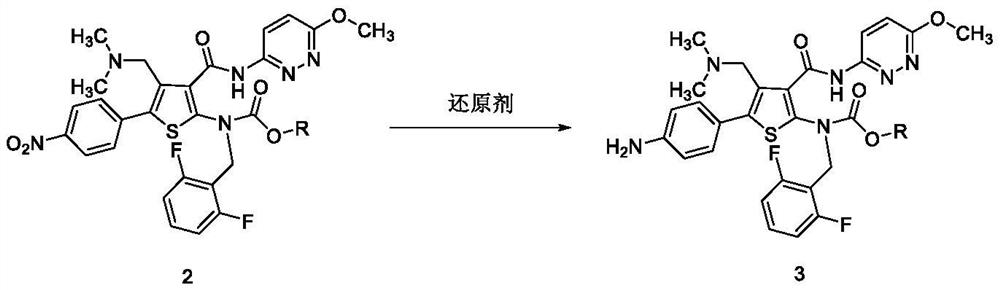
Preparation method of Relugolix and intermediate compounds
InactiveCN112745304AMild reaction conditionsHigh response controllabilityOrganic chemistryMethoxyamineMeth-
The invention provides a novel preparation method of Relugolix and two novel intermediate compounds. The preparation method comprises the following steps: reducing an existing compound 2 to obtain an amino compound 3, then, carrying out a condensation reaction on the compound 3 and methoxyamine or a salt thereof to obtain a compound 4, and finally, carrying out a cyclization reaction on the compound 4 to obtain a target compound 1. The preparation method is mild in reaction condition and high in reaction controllability; and the method has the advantages of few impurities, no ring-opening and demethylation impurities, good purity, simple post-treatment, high yield, environmental protection, economy and good industrial application prospect.
Owner:SHANGHAI DUDE MEDICAL SCI & TECH CO LTD
Method for safely preparing pure N, O-dimethylhydroxyamine hydrochloride
InactiveCN104478757ASpeed up decompositionImprove conversion rateOrganic chemistryHydroxylamineHydroxylamine Hydrochloride
The invention relates to a method for safely preparing N, O-dimethylhydroxyamine hydrochloride. The method comprises the steps: dissolving rough N, O-dimethylhydroxyamine hydrochloride into water; feeding an alkaline substance to neutralize to fractionate out methoxyamine and N, O-dimethyl hydroxylamine; then feeding acetone or butanone; preparing ketoxime ethers through acetone or butanone and methoxyamine; then feeding hydrochloric acid; heating and refluxing at a normal pressure to continuously separate out generated non-condensed unidentified gas; performing the operation of distilling off water to replace the refluxing operation after detecting relatively small chromatographic value of methoxyamine in an aqueous liquid; dissolving a water-distilled solid with methyl alcohol; filtering out a methyl alcohol solution for recrystallizing; concentrating and cooling to separate out a crystal; and then centrifuging and drying to obtain the pure N, O-dimethylhydroxyamine hydrochloride. According to the method, ketoxime ethers can be decomposed under an acidic heating condition, so that methoxyamine and N, O-dimethyl hydroxylamine in the heating state are in forms of hydrochloride instead of a free state, and as a result, the safety in production and purification can be ensured.
Owner:YANTAI AODONG CHEM MATERIAL
Liquid phase synthesis process of oxime strain ester by poly-ethandiol
InactiveCN1560028ASimplify separation and purification stepsBroad mindOximes preparationPolyethylene glycolAcetophenone
The invention discloses a method of synthesizing trifloxystrobin by using liquid phase polyglycol: (a) with the help of hydroxyl on the olyglycol to make condensation reation with o-methyl benzoylformic acid, loading the o-methyl benzoylformic acid on the olyglycol, so as to obtain 2-(2'-methylphenyl)-2- polyglycol carboxy acetate; (b) making the product obtained in step (a) react with methoxy amine and acid salts, obtaining 2-(2'-methylphenyl)-2- polyglycol carboxy acetate-O-methyl ketone oxime; (c) bromizing the product obtained in step (b) to obtain 2-(2'-bromomethylphenyl)-2- polyglycol carboxy acetate-O-methyl ketone oxime; (d) on basic condition, condensing the compound obtained in step (c) with meta-trifluoromethyl acetophenone oxime to obtain 2-[1'-{[(3'-trifluoromethylphenyl)-ethyl-imine]O}-O-tolyl]-2-polyglycol carboxy acetate-O- methyl ketone oxime; (e) making ester exchange reaction of the compound obtained in step (d) with methanol to obain the trifloxystrobin. On the basis of not changing properties of trifloxystrobin, it simplifies the step of separating and purifying in reacting course, reducing the cost.
Owner:TONGJI UNIV
Preparation method of 2-methoxyimino-2-furyl ammonium acetate
The invention provides a preparation method of 2-methoxyimino-2-furyl ammonium acetate. The preparation method includes: enabling a compound of a structure shown as a formula (I) to react with methoxyamine to obtain reaction liquid containing the compound of a structure shown as a formula (II); adding alkali into the reaction liquid to obtain reaction liquid containing a compound of a structure shown as a formula (III); adding ammonium salt into the reaction liquid containing the compound of the structure shown as the formula (III) to obtain 2-methoxyimino-2-furyl ammonium acetate. A target product is obtained by selecting the compound of the structure shown as the formula (I) as a starting raw material, enabling the starting raw material to react with methoxyamino acid and hydrolyzing and ammonifying a reaction product, preparation from the starting raw material to the final product can be completed in a same reaction system, an intermediate of the reaction does not need to be separated, and yield and purity of the reaction are high.
Owner:四平市精细化学品有限公司
Method for recovering methoxyamine from furan ammonium salt wastewater
ActiveCN110003043AAccelerate evaporationBring out the guaranteeOrganic chemistryChemical industryFuran
The invention belongs to the technical field of medical chemical industry, and particularly relates to a method for recovering methoxyamine from furan ammonium salt wastewater. The method comprises the following steps of: adding calcium carbonate into the furan ammonium salt wastewater, and regulating pH to 3.0-3.5 to obtain calcium hydrogen phosphate salt emulsion; adding a flocculant into the obtained calcium hydrogen phosphate salt emulsion for flocculation sedimentation; filtering to obtain calcium hydrogen phosphate salt solid and filtrate; concentrating the filtrate under reduced pressure to evaporate water; filtering to remove precipitated sodium chloride to obtain the filtrate; regulating the pH of the filtrate to 12-14; raising the temperature; introducing nitrogen into the filtrate; carrying out nitrogen purge distillation; and receiving steam carried by the nitrogen to obtain a methoxamine aqueous solution. By the adoption of the method for recovering the methoxyamine from the furan ammonium salt wastewater, three salts in the furan ammonium salt wastewater can be effectively separated, recycling of three raw materials of methoxyamine, phosphoric acid and sodium chloridefor producing the furan ammonium salt is achieved, production cost of the furan ammonium salt is further reduced, distillation waste residue is reduced and economic benefit is improved.
Owner:SHANDONG JINCHENG PHARMACCUTICAL CHEM CO LTD
Preparation method for celecoxib isomer
InactiveCN102617474ALow costSimple post-processingOrganic chemistryPhenylsulfonamidePhenylhydrazine hydrochloride
The invention discloses a preparation method for celecoxib isomer, which includes the steps: firstly, preparing a crude product of 4, 4, 4-trifluoro-3-methoxyimino-1-(4-cresyl)-1-butanone from 4, 4, 4-trifluoro-1-(4-cresyl)-1, 3-butanedione with methoxylamine hydrochloride serving as ammoniation protective reagent; and secondly, adding p-amino sulfonyl phenylhydrazine hydrochloride into the prepared crude product, dissolving with ethyl acetate and acetic acid, obtaining a crude product after heating and backflow, and recrystallizing to obtain a pure product of 4-[3-(4-methylphenyl)-5-(trifluoromethyl)-1H-pyrazolyl] benzene sulfonamide, namely, the celecoxib isomer, which is white or almost white crystalline powder in appearance. The preparation method has the advantages of low cost, simplicity in post-processing, high yield and high selectivity.
Owner:STONE LAKE PHARMA TECH
Synthesis method for forming indazole derivative by activating hydroxylamine
InactiveCN102532027AEfficient synthesisEasy to purifyOrganic chemistryChemical synthesisHydroxylamine
The invention relates to a synthesis method for forming indazole derivative by activating hydroxylamine, and belongs to the field of a chemical synthesis method. The method comprises the following steps of: adding methoxylamine hydrochloride and potassium carbonate into fluoro aromatic formaldehyde serving as a raw material and tetrahydrofuran serving as a solvent; reacting at normal temperature for a night; filtering, concentrating and quantifying to obtain an intermediate compound fluoro aromatic methoxyl oxime; dissolving the intermediate into tetrahydrofuran; adding hydrazine hydrate; refluxing for a night to obtain the methoxyl substituted indazole derivative; and heating, refluxing and removing the methoxyl under the acidic condition to obtain high-yield indazole derivative. According to the method, the hydrazine hydrate with low toxicity and low danger serves as a reaction agent, so the method is high in yield and convenient for purification and can be applied to amplification of industrialized production.
Owner:常州协丰新材料科技有限公司
One-step method for synthesizing N-methyl-N-methoxyamide
InactiveCN101514171AEfficient synthesisLower synthesis costSulfonic acid amide preparationMethoxyamineAfter treatment
The invention provides a one-step method for synthesizing N-methyl-N-methoxyamide, comprising the steps of: taking methylene dichloride or tetrahydrofuran as a solvent, reacting carboxylic acid with triphenylphosphine, N-bromosuccimide and N-methyl-N-methoxyamine to generate relevant N-methyl-N-methoxyamide. The invention is simple in reaction procedure, easily-available in raw materials, mild in reaction conditions, environmentally-friendly, low in synthesis cost, high in efficiency and yield and easy in after treatment as well as can efficiently synthesize various chiral or achiral N-methyl-N-methoxyamide.
Owner:NORTHWEST NORMAL UNIVERSITY
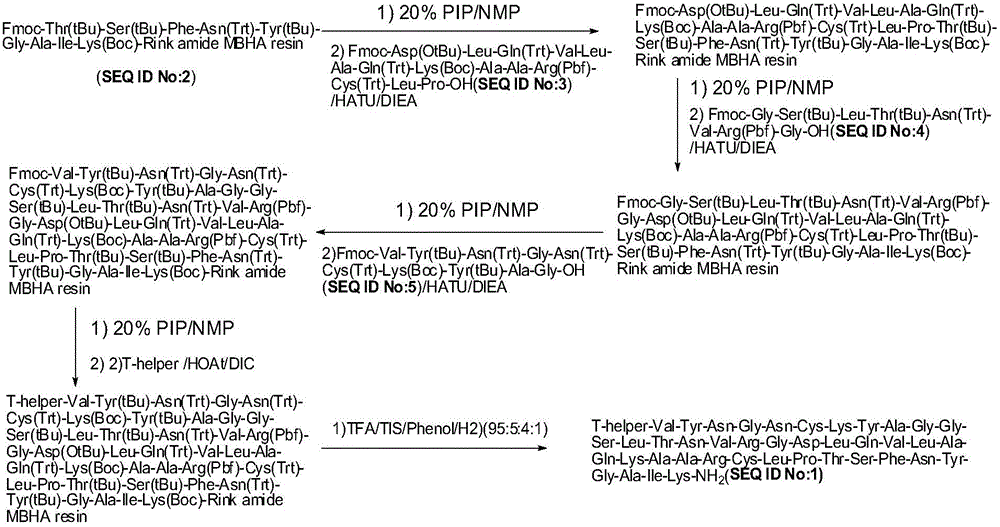
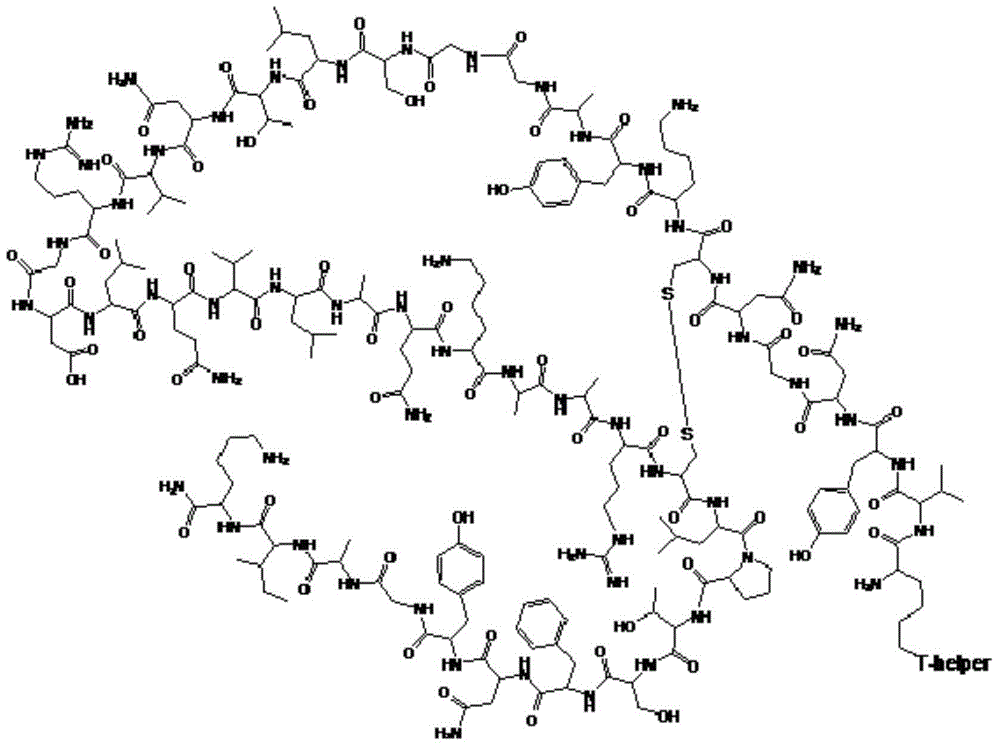
Method for preparing pig O-type foot-and-mouth disease synthetic peptide antigen 2800 by using solid-phase fragment method
ActiveCN104311673AAvoid the risk of wrong connectionIncrease production capacityPeptide preparation methodsHybrid peptidesPeptide antigenCoating system
The invention provides a method for preparing pig O-type foot-and-mouth disease synthetic peptide antigen 2800 by using a solid-phase fragment method. The method comprises the following steps: by taking resin as an initial raw material, sequentially connecting amino acid with 9-fluorene methoxycarbonyl group protection, preparing a protected peptide fragment, sequentially removing 9-fluorene methoxycarbonyl groups in the period, performing peptide connection reaction by using a condensating agent, and cutting by using diluted acid or weak acid so as to obtain the protected peptide fragment; gradually connecting the fragment with 4-(4'-dimethoxy-9-fluorene methoxyamine methyl)-phenoxymethyl resin, and further connecting with T-auxiliary cell epitope; cutting off the protection groups and the resin by using acid so as to obtain synthetic peptide antigen 2800 coarse peptide; purifying by using ion exchange and a tangential flow membrane coating system, and concentrating to remove micromolecules and salt, thereby obtaining the synthetic peptide antigen 2800. The method provided by the invention has the characteristics of being stable in process, short in production period, convenient in obtaining raw / auxiliary materials, small in waste, low in production cost, high yield and the like.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP
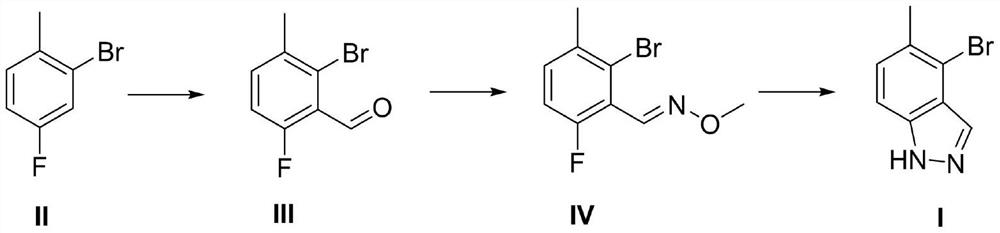
Preparation method of 4-bromo-5-methyl-1H-indazole
InactiveCN112321510AReduce pollutionEasy to handleOrganic chemistryLithium diisopropylamidePotassium carbonate
The invention discloses a preparation method of 4-bromo-5-methyl-1H-indazole, wherein the preparation method comprises the specific steps: (1) reacting a compound (II) in the presence of lithium diisopropylamide to generate a lithium reagent, and reacting the lithium reagent with dimethylformamide to generate a compound (III); (2) reacting the compound (III) with methoxyamine hydrochloride and potassium carbonate to obtain a compound (IV); and (3) cyclizing the compound (IV) under the participation of hydrazine hydrate to generate a compound (I). The preparation method is higher in yield.
Owner:无锡双启科技有限公司
Method for synthesizing methoxy amine hydrochlorate
InactiveCN101503375BEmission reductionMild reaction conditionsOrganic chemistryEvaporation2-butanone oxime
The invention discloses a synthesizing method of methoxy amine hydrochloride. Butanone oxime, water, sodium hydroxide, phase transfer catalyst are added in a reaction container; the temperature is decreased to 0-15 DEG C; a methylating reagent begins to be added; then, reaction is carried out at a temperature of 5-120 DEG C for 3-6 h; standing and layering are carried out so that an organic layerO-methyl-2-diacetylmonoxime ether and a water layer are separated; then, the O-methyl-2-diacetylmonoxime ether in the water layer is distilled and recovered under the normal pressure; the separated O-methyl-2-diacetylmonoxime ether is mixed with the distilled and recovered O-methyl-2-diacetylmonoxime ether and then with hydrochloric acid solution with a mass percentage of 30-35 percent; the mixed solution undergoes feeding and rectification at the middle of a rectifying column; butanone and methanol are recovered by the column top; methoxy amine hydrochloride solution is recovered by the column bottom; and the methoxy amine hydrochloride solution also undergo evaporation and dehydration by heating so as to obtain a hydrochloride concentrated solution thereof. The synthesizing method is simple, generates a small amount of three wastes and improves the operation environment and the yield rate.
Owner:宁波四明化工有限公司 +1
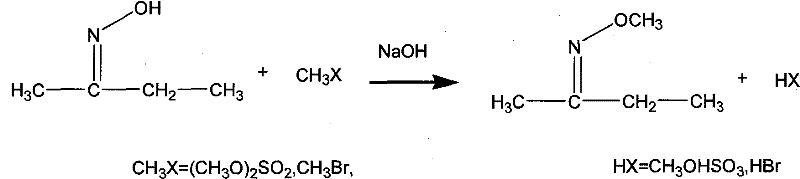
Method for preparing methoxyamine, method for preparing methoxyamine hydrochloride
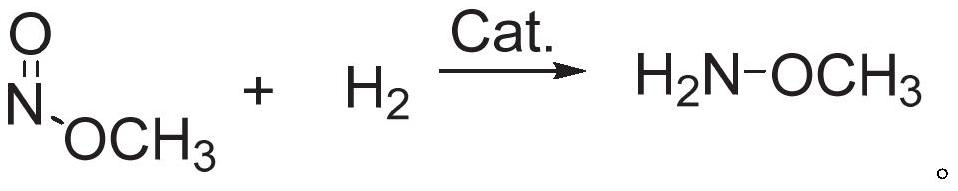
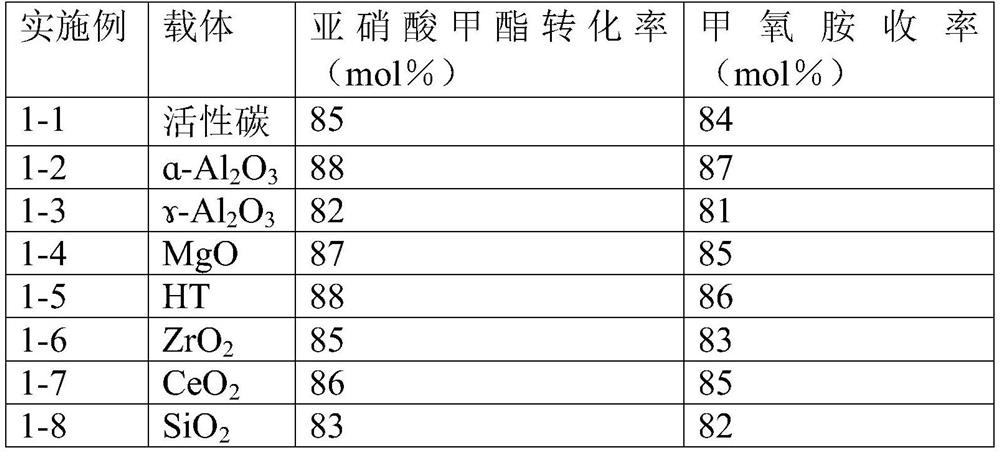
ActiveCN112125822BImprove conversion efficiencyEasy to operateOrganic chemistryPtru catalystMethyl nitrate
The present application discloses a method for preparing methoxyamine, which at least includes: contacting a raw material gas containing methyl nitrite and a reducing agent with a reduction reaction catalyst in a reactor to perform a reduction reaction to obtain methoxyamine. The method can fully utilize the important intermediate methyl nitrite in the coal-to-ethylene glycol process, and the conversion rate of the methyl nitrite is high. The present application also provides a preparation method of methoxyamine hydrochloride obtained by the above method as raw material.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
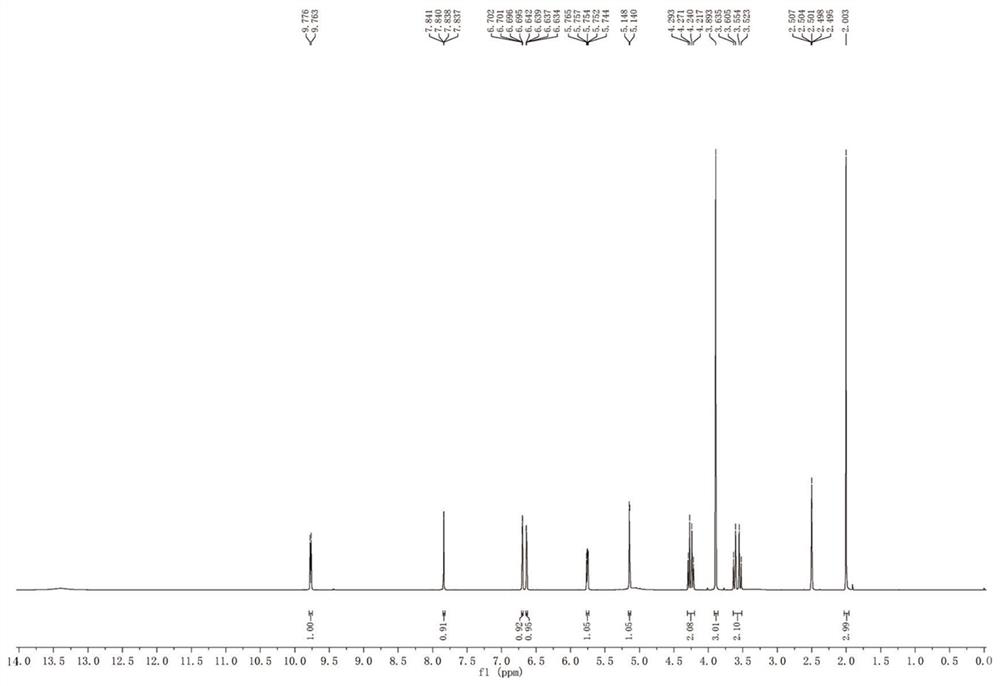
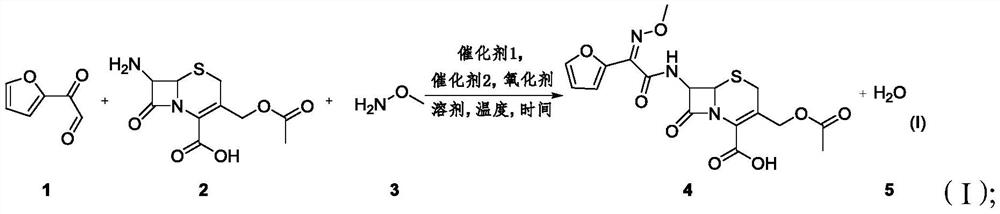
Method for synthesizing 3-decarbamoyl-acetyl-cefuroxime acid compound
ActiveCN112679527AThe process steps are simpleReduce cost pressureOrganic chemistryFuranPtru catalyst
The invention provides a method for synthesizing a 3-decarbamoyl-acetyl-cefuroxime acid compound. The method comprises the following steps: taking 2-(2-furyl)-2-oxo-acetaldehyde, 7-aminocephalosporanic acid and methoxyamine as raw materials; reacting the raw materials in a reaction solvent under the combined action of a first catalyst, an oxidizing agent and a second catalyst; and carrying out chromatography to obtain the 3-deaminobenzoyl acetyl cefuroxime acid compound. By using the method, the process steps are simple, the byproduct is mainly water, a large amount of other wastes do not exist, and the subsequent treatment cost and the environmental protection pressure are low.
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
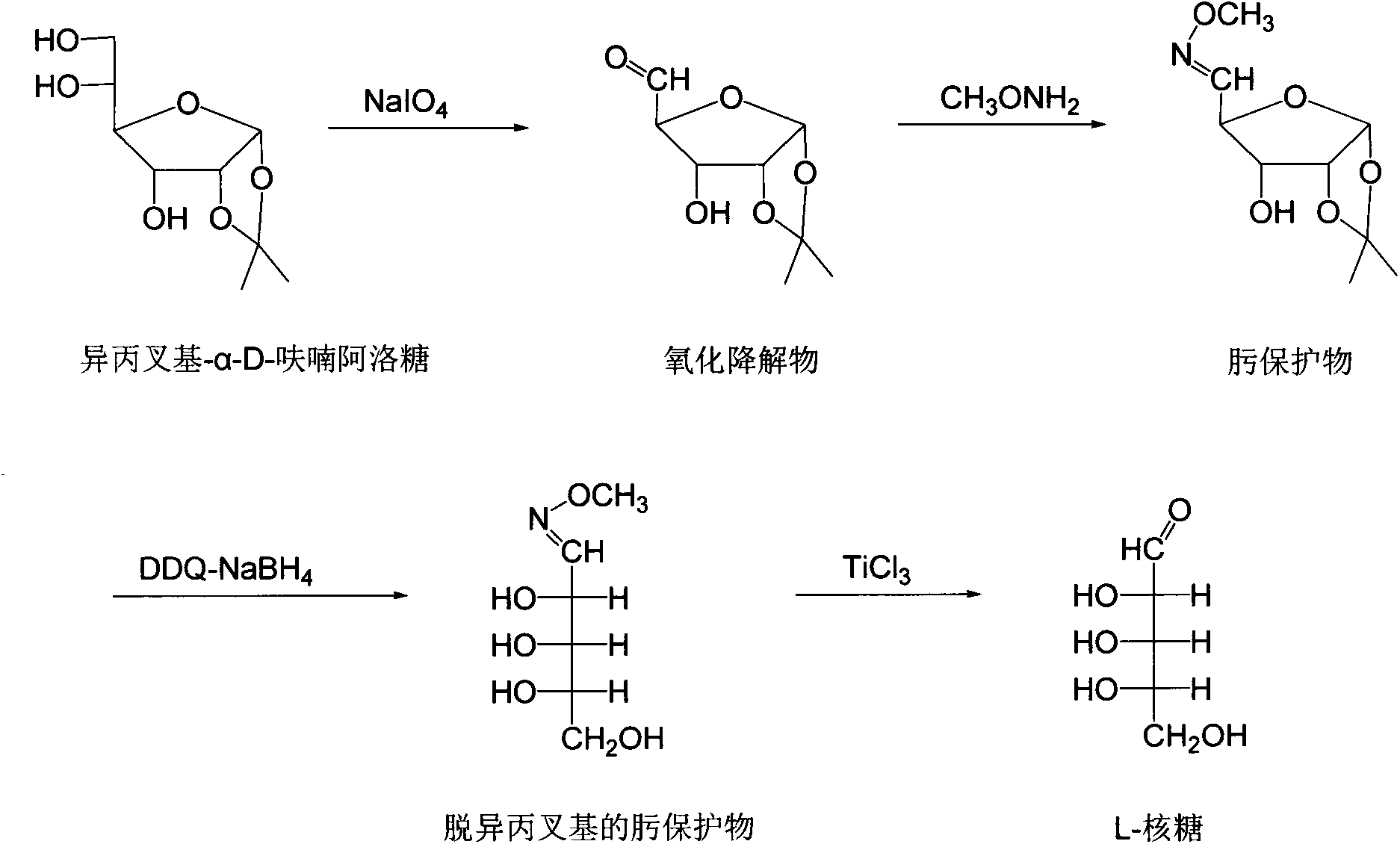
Chemical preparation method of L-ribose
InactiveCN103709207ASimple and fast operationLow costSugar derivativesSugar derivatives preparationSodium bicarbonateFuran
The invention relates to a chemical preparation method of L-ribose. The chemical preparation method is characterized by comprising the following steps: formulating mesityl-alpha-D-furan allose, sodium bicarbonate and distilled water into a reaction system, and adding sodium periodate for reaction at a certain temperature to obtain oxidative breakdown products; heating the oxidative breakdown products with a methanol solution of methoxyamine for a reflux reaction to obtain an oxime protected object; reacting the oxime protected object and dichlorodicyanobenzoquinone with acetonitrile-aqueous solution, then adding sodium borohydride liquid for reaction to obtain a demesityl oxime protected object; then reacting the demesityl oxime protected object with a tetrahydrofuran solution of titanium trichloride to obtain the L-ribose.
Owner:TIANJIN POLYTECHNIC UNIV
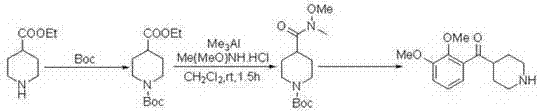
Preparation method of N-methoxy-N-methyl-1-p-toluenesulfonyl piperidine-4-amide
InactiveCN102491941BImprove responseShort reaction cycleOrganic chemistryChemical synthesisCarboxylic acid
The invention discloses a preparation method of N-methoxy-N-methyl-1-p-toluenesulfonyl piperidine-4-amide, which is an intermediate for producing a medicine MDL-100907 (Volinanserin), belonging to the technical field of chemical synthesis. In the method, PCl3 and N-methyl-N-methoxy amine are adopted as amidation reagents and directly act with 1-toluenesulfonyl piperidine-4-carboxylic acid to prepare the N-methoxy-N-methyl-1-p-toluenesulfonyl piperidine-4-amide by using a one-pot process, so that the step of separating and purifying phosphoramidite as the amidation reagent is omitted, the problem that the phosphoramidite is instable and is difficult to separate is solved, the reaction is greatly simplified and the reaction period is shortened; raw materials and various reagents are all cheap and easily obtainable, the cost is low, a post-processing process is simple and the yield is high; and the adopted raw materials are nontoxic, and a production process is pollution-free and environment-friendly, so that favorable conditions are created for industrialized scale production and commercialization of products.
Owner:NORTHWEST NORMAL UNIVERSITY
Anti solid tumor medicine composition
InactiveCN1279980CInhibitory activityAntineoplastic agentsPharmaceutical active ingredientsHydroxylamineWhole body
Owner:南京天一药业有限公司
Method for synthesizing (z)‑2‑(α‑methoxyimine) ammonium furoacetate
The invention belongs to the technical field of medical intermediate preparation, and particularly relates to a method for synthesizing (Z)-2-(alpha-methoxyimino)furanylacetic acid ammonium. The method comprises the following steps that 1, 2-oxo-2-furanylacetic acid and heavy metal salt are dissolved in water, an aqueous methoxyamine solution or aqueous methoxyamine salt solution is added at the temperature of 0 DEG C-10 DEG C, and the pH is regulated to 2.5-3.5; 2, heat preservation is performed for 2-7 h at the temperature of 5 DEG C-10 DEG C, and a 2-(alpha-methoxyimino)furanylacetic acid solution is obtained; 3, the pH of the 2-(alpha-methoxyimino)furanylacetic acid solution is regulated to 0.1-1.5 by adopting inorganic acid, the temperature is controlled at 15 DEG C-25 DEG C, extraction is performed through organic solvent, and organic phases are combined; 4, ammonia gas or liquid ammonia is introduced into the organic phases at the temperature of 0 DEG C-10 DEG C, the pH is regulated to 6.5-7.5, heat preservation is performed for 0.5-1.5 h to obtain a crude product, and after decoloration, concentration and crystallization are performed, the product is obtained. The method is high in yield and good in product quality.
Owner:SHANDONG JINCHENG PHARMACCUTICAL CHEM CO LTD
Preparation method of Iguratimod intermediate
PendingCN114539104AEasy to getPromote environmental protectionOrganic compound preparationSulfonic acid amide preparationP-nitroanisolePtru catalyst
The invention discloses a preparation method of an Iguratimod intermediate, which comprises the following steps: by taking p-nitroanisole as a raw material, carrying out substitution nucleophilic substitution (VNS) on p-nitroanisole and methoxyamine hydrochloride in the presence of a copper salt catalyst and an acid-binding agent to generate 5-methoxy-2-nitroaniline (compound II); the synthesis method comprises the following steps: carrying out a nucleophilic substitution reaction on 5-methoxy-2-nitroaniline (compound II) and methanesulfonyl chloride to generate a compound III, etherifying the compound III and phenol under the catalysis of a copper salt to generate N-(5-methoxy-2-phenoxy phenyl) methane sulfonamide (compound IV), and reagents used in the synthesis process are non-highly toxic products and are easy to obtain; no iron powder is used in the reaction process, so that iron mud which is harmful to the environment is not generated, and the environmental protection property is high; the reaction operation difficulty is small, the safety is high, and a foundation is laid for industrial preparation of Iguratimod drugs.
Owner:常州佳德医药科技有限公司
Synthetic process of furan ammonium salt
The invention belongs to the technical field of medical intermediate preparation, and particularly relates to a synthetic technology of furan ammonium salt. The synthetic technology comprises the steps that 2-acetyl furan is used as a raw material to form furanone acid through oxidative synthesis at first, then esterification is performed, the product reacts with methoxyamine to be synthesized into a methoxy group oximation product, the methoxy group oximation product is hydrolyzed and then reacts with an alcohol amine solvent, and the furan ammonium salt is obtained. Due to the fact that the furanone acid is esterified at first, the carbonyl activity is improved, and more oximation products are promoted to be converted into cis-form products. The content of the obtained anti-form furan ammonium salt is only 5-8%; compared with a traditional furan ammonium salt production method, the content of the anti-form furan ammonium salt is obviously reduced without lowering productivity.
Owner:SHANDONG JINCHENG PHARMACCUTICAL CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com