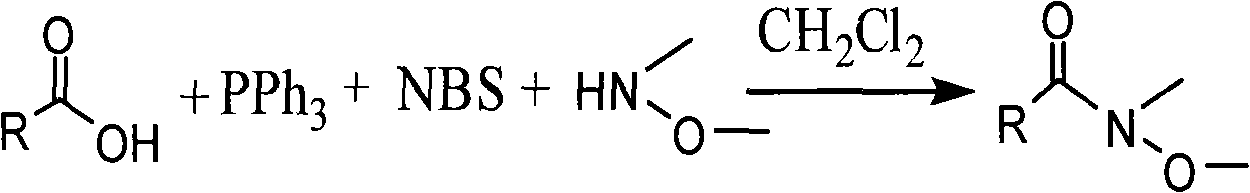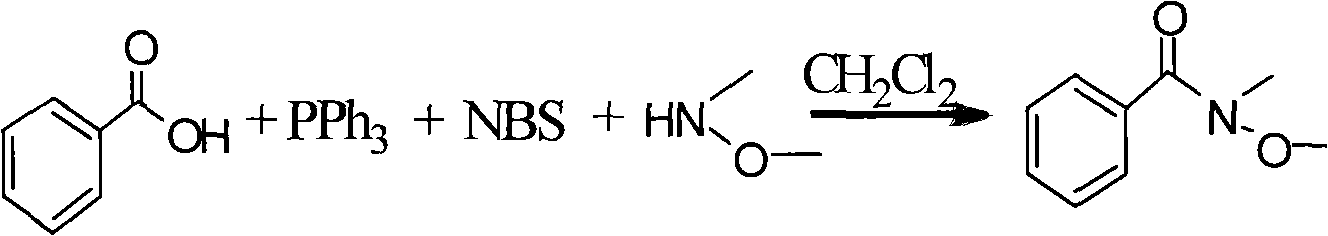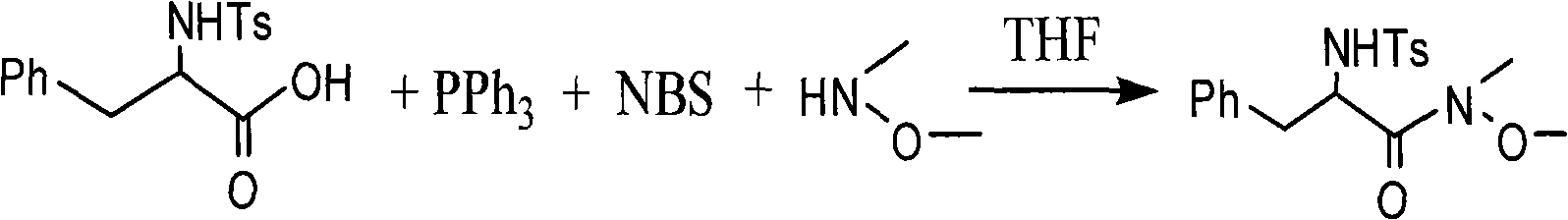One-step method for synthesizing N-methyl-N-methoxyamide
A technology of methoxy amide and methoxy amine, which is applied in the preparation of sulfonic acid amide, organic chemistry, etc., can solve the problems of difficult residues or by-products, expensive deoxidizing and fluorinating agents, and wasteful yield of raw materials. Ease of handling, low synthesis cost, effect of reaction time period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The synthesis of embodiment 1, N-methyl-N-methoxybenzamide
[0019] In a 50mL two-neck flask, add 4mL of dichloromethane, cool down to about 0°C, add 1mmol of benzoic acid, 1mmol of triphenylphosphine and 1.1mmol of N-bromosuccinimide under stirring; stir for 10 minutes Then add 1.7mmol of N-methyl-N-methoxylamine, after reacting for 30min, add NaHCO 3 The saturated solution was quenched for 30 min, and the organic phase was separated; the aqueous phase was extracted with dichloromethane, and the anhydrous MgSO 4 Drying, column chromatography to obtain the target compound - N-methyl-N-methoxybenzamide. The yield was 81%.
[0020] Its reaction formula is as follows:
[0021]
[0022] Target compound N-methyl-N-methoxybenzamide by IR, 1 HNMR, 13 CNMR detects that its product is a pure target compound, and the data are as follows:
[0023] IRv(neat) / cm -1 3061, 2968, 2933, 2819, 1644, 1576, 1451, 1416, 1377, 1213, 982, 787, 707;
[0024] 1 H NMR (400MHz, CDCl ...
Embodiment 2
[0026] Embodiment 2, the synthesis of N-methyl-N-methoxyl group-2-(N-p-methylbenzenesulfonyl) phenylpropanamide
[0027] In a 50mL two-necked flask, add 4mL tetrahydrofuran, cool down to about 0°C, and mix 1mmol (N-p-toluenesulfonyl)phenylpropionic acid, 1mmol triphenylphosphine and 1.1mmol N-bromobutylene under stirring Add imide; stir for 10 minutes, add 1.7mmol of N-methyl-N-methoxyamine, react for 1h, add NaHCO 3 The saturated solution quenched the reaction for 30 min; the organic phase was separated, the aqueous phase was extracted with dichloromethane, anhydrous MgSO 4 Drying and column chromatography yielded the target compound—N-methyl-N-methoxy-2-(N-p-methylbenzenesulfonyl)phenylpropanamide. The yield was 88%.
[0028] Its reaction formula is as follows:
[0029]
[0030] The target compound N-methyl-N-methoxy-2-(N-p-methylbenzenesulfonyl) phenylpropanamide was subjected to IR, 1 HNMR 13 CNMR detection, its product is a pure product, the data is as follows:
...
Embodiment 3
[0034] Embodiment 3: the synthesis of N-methyl-N-methoxy-3,5,5-trimethylhexanamide
[0035] Steps: In a 50mL two-neck flask, add 4mL tetrahydrofuran, cool down to about 0°C, stir 1mmol 3,5,5-trimethylhexanoic acid, 1mmol triphenylphosphine and 1.1mmol N-bromobutanedi Add imide to it; stir for 10 minutes, add 1.7mmol of N-methyl-N-methoxyamine, react for 3h, add NaHCO 3 The saturated solution was quenched for 30 min, the organic phase was separated, the aqueous phase was extracted with dichloromethane, anhydrous MgSO 4 Drying and column chromatography gave the target compound - N-methyl-N-methoxy-3,5,5-trimethylhexanamide. The yield was 99%. Its reaction formula is as follows:
[0036]
[0037]The target compound N-methyl-N-methoxy-3,5,5-trimethylhexanamide by IR, 1 HNMR, 13 CNMR detection, its product is a pure product, the data is as follows:
[0038] IR v(neat) / cm -1 3319, 2955, 2934, 1668, 1465, 1412, 1379, 1247, 1177, 1116, 1004, 940;
[0039] 1 H NMR (400MHz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com