Synthesis method for forming indazole derivative by activating hydroxylamine
A synthesis method and derivative technology, applied in the direction of organic chemistry, etc., can solve the problems of harsh reaction conditions and narrow application range of reaction conditions, and achieve the effect of simple and convenient purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] A kind of synthetic method that forms indazole derivative by the activation of hydroxylamine, described indazole derivative is shown in formula (I), and synthetic method comprises the steps:
[0038] a, with o-fluoro aromatic formaldehyde as shown in formula (II) as raw material, tetrahydrofuran as solvent, add methoxylamine hydrochloride and potassium carbonate, react overnight at normal temperature;
[0039] b. The solution obtained in step a is filtered, concentrated, and quantitatively obtained as an intermediate compound such as o-fluoroaromatic methoxyxime shown in formula (III);
[0040] c. Dissolve the intermediate compound (III) obtained in step b in tetrahydrofuran, add hydrazine hydrate, and reflux overnight to obtain a methoxy-substituted indazole derivative as shown in formula (IV);
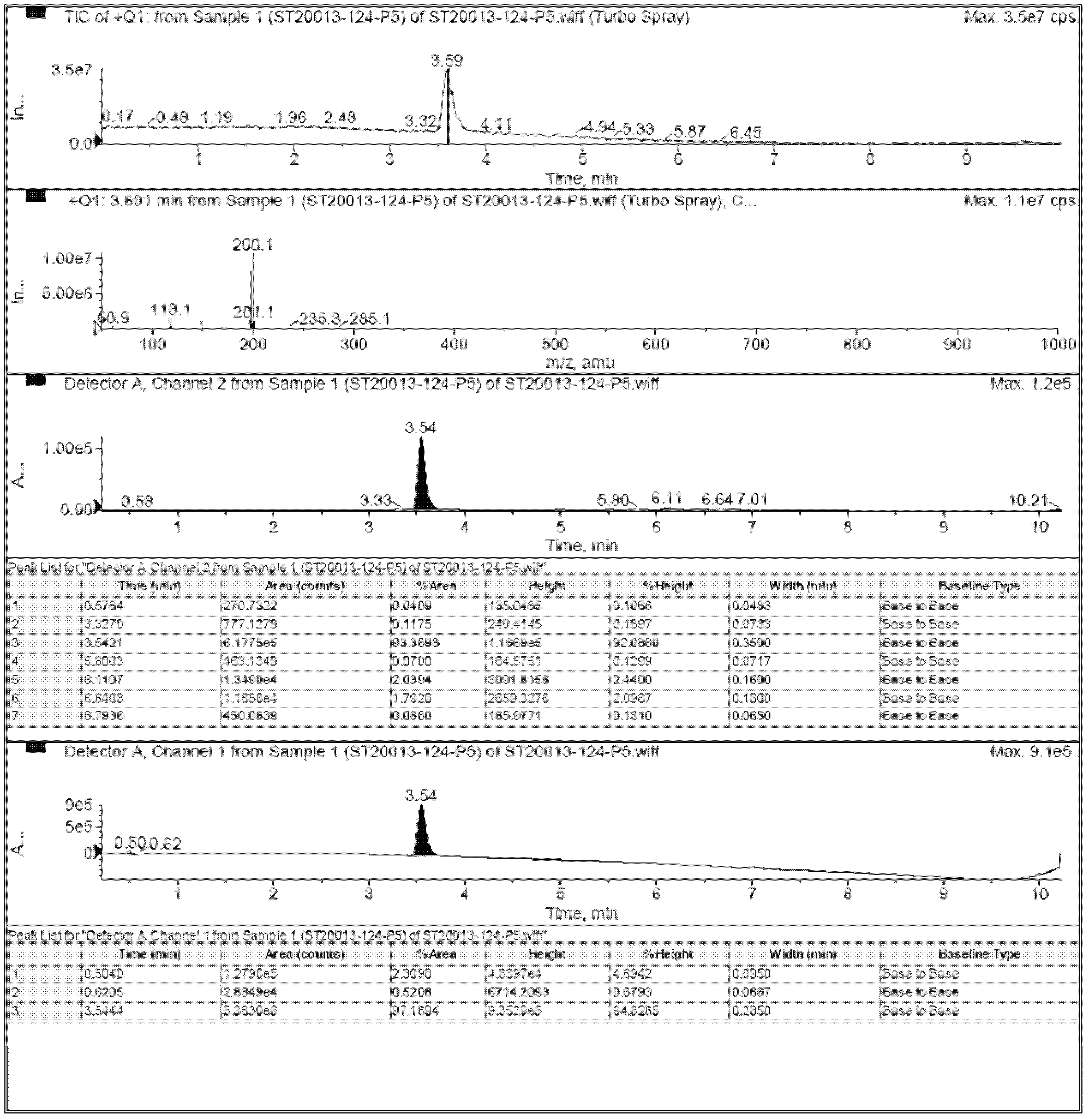
[0041] d. The methoxy-substituted indazole derivative shown in (IV) obtained in step c is heated to reflux under acidic conditions to remove the methoxy group to obtain the in...
Embodiment
[0051] The present invention has carried out concrete experiment, and its experimental process and result are as follows:
[0052]
[0053] First step reaction: A→B
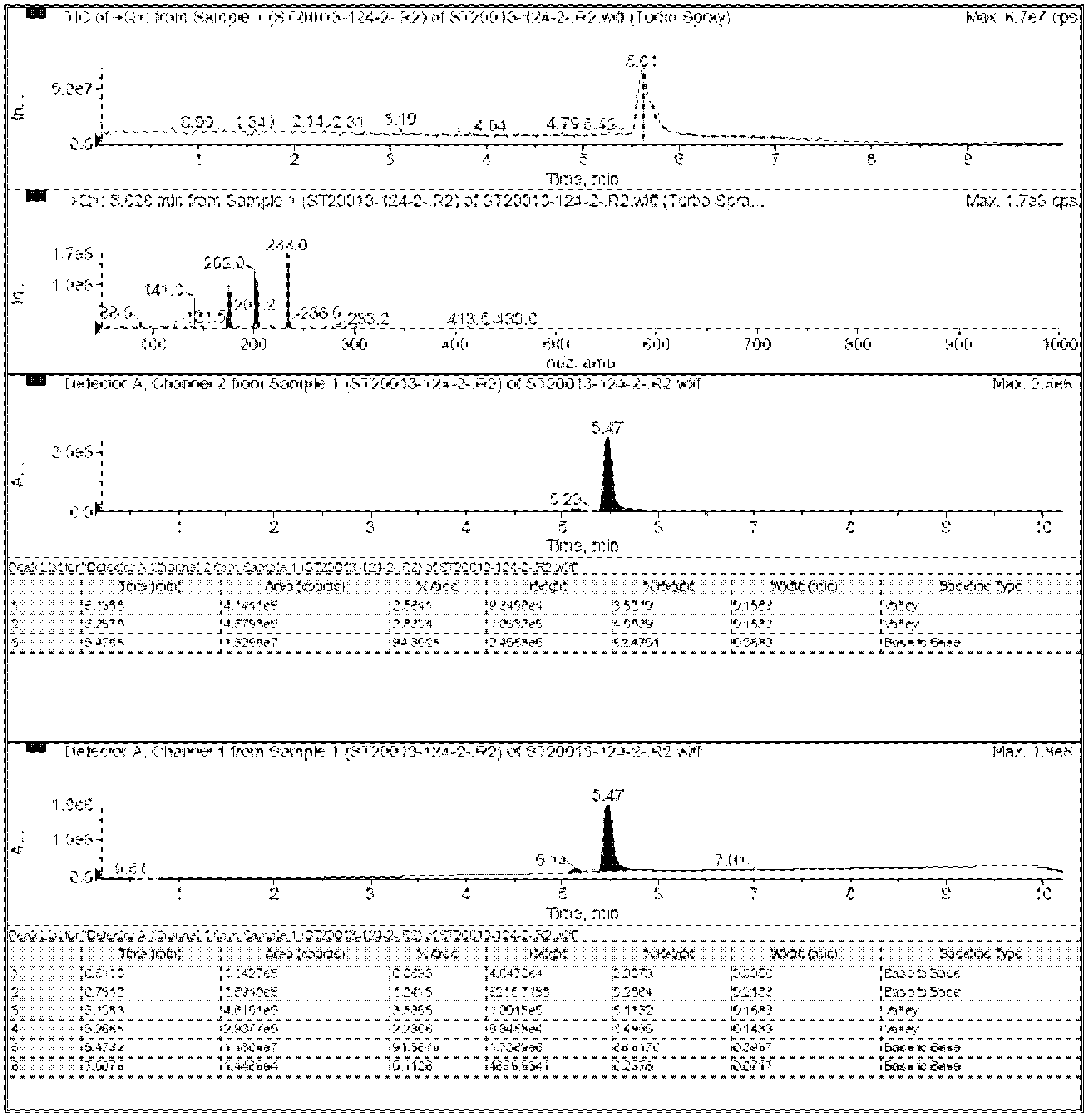
[0054] 2-Bromo-5-fluoro-4-carbaldehydepyridine (0.98 mol, 200.0 g), methoxylamine hydrochloride (0.98 mol, 81.85 g), and potassium carbonate (1.08 mol, 149.3 g) were added to 1.5 L of THF The solution was stirred at room temperature for 13 hours. The reaction solution was filtered, and the filtrate contained product B, and then the filtrate was concentrated to 1 liter, such as figure 1 Shown is the LCMS profile of the first step reaction.
[0055] Second step reaction: B→C
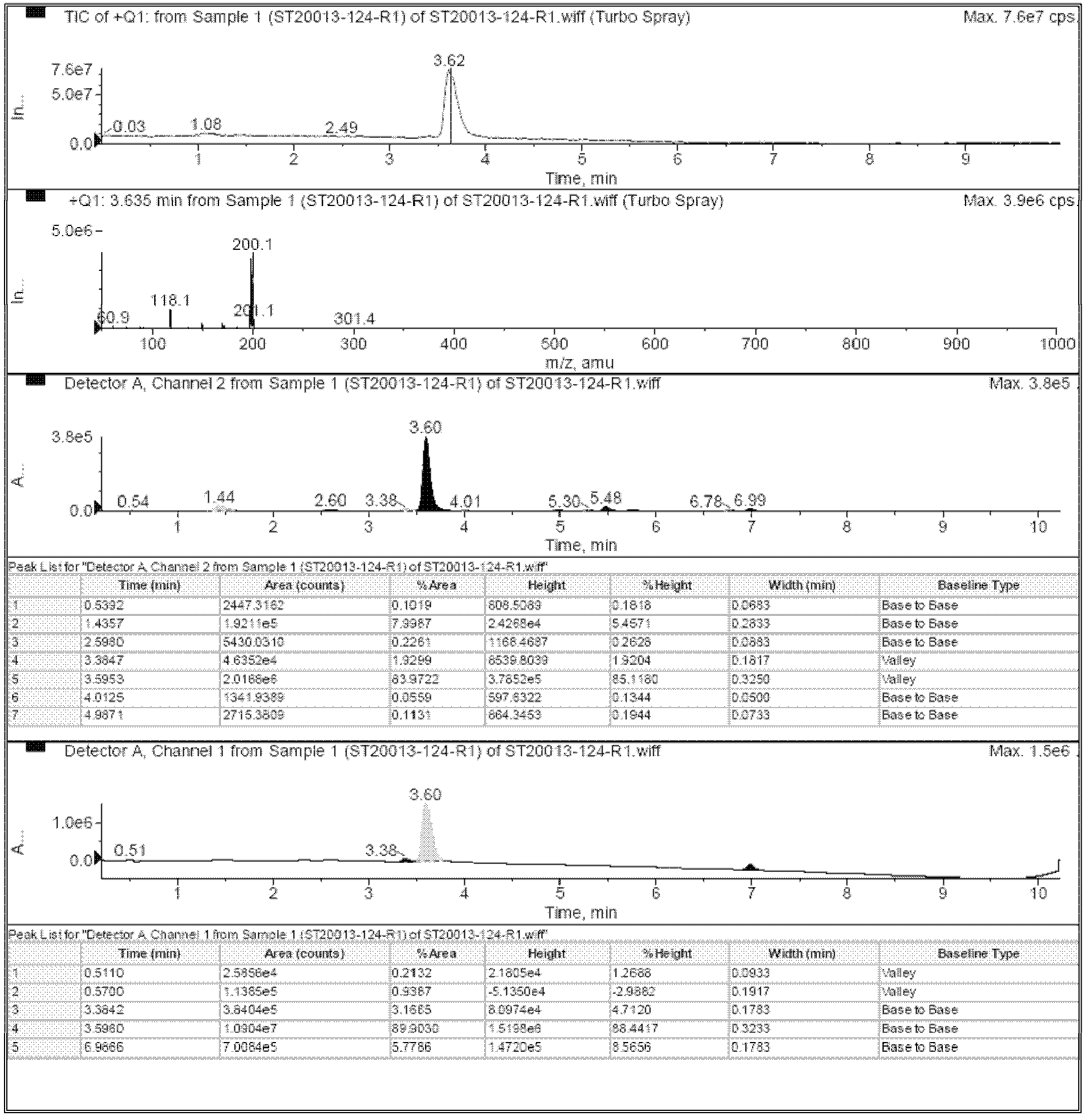
[0056] Add 400 ml of hydrazine hydrate to the concentrated filtrate in the first step reaction, and reflux overnight. The reaction solution was directly concentrated by a vacuum pump to 500 ml, and 1 liter of distilled water was slowly added to the concentrated solution with constant stirring. A large amount of precipitate was precipit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com