Preparation method of Iguratimod intermediate
A technology for intermediates and compounds, which is applied in the field of chemical drug synthesis, can solve the problems of difficult handling of iron sludge, large environmental pollution, and difficulty in obtaining raw materials, and achieves the effects of high environmental protection, high safety and less difficulty in reaction operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] N-(5-methoxy-2-phenoxyphenyl)methanesulfonamide (compound IV) is prepared by the preparation method of an Iguratimod intermediate of the present invention, and the specific operation is as follows:
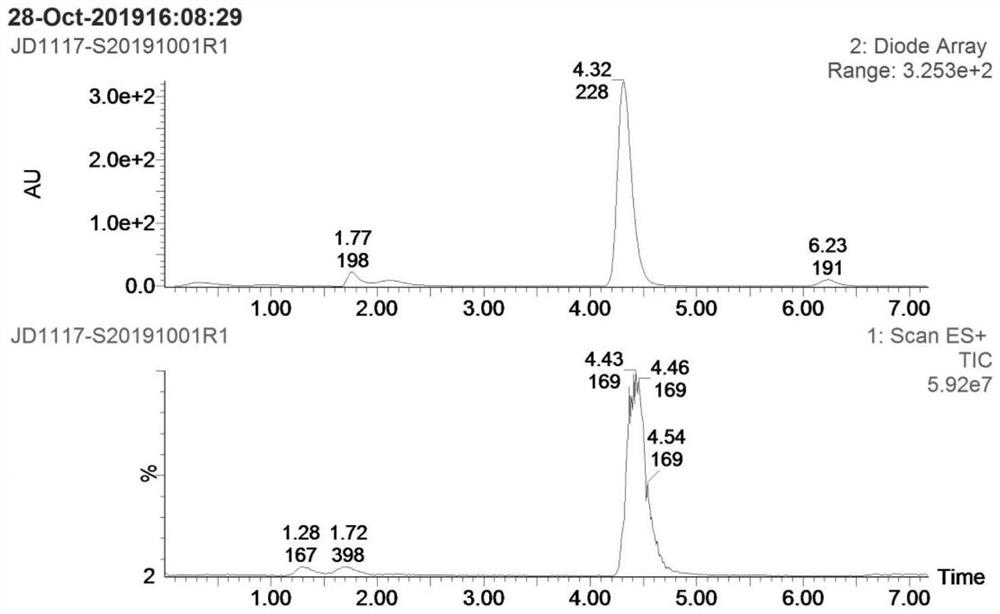
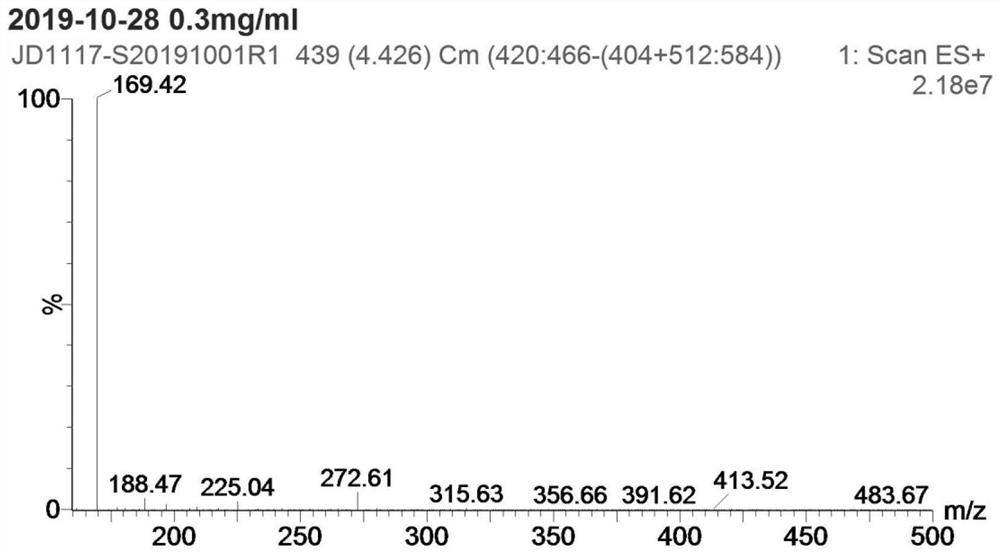
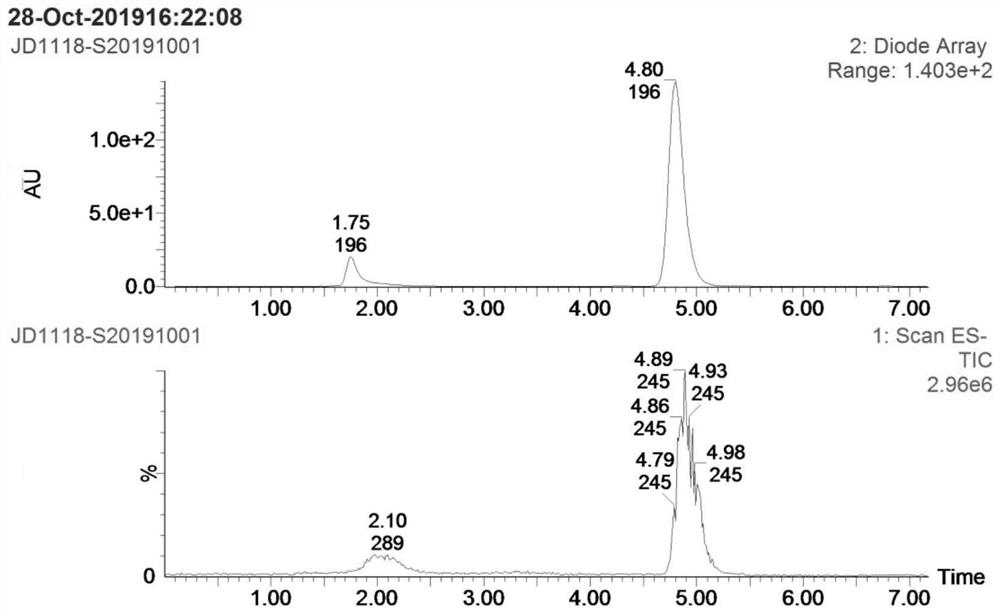
[0028] Step S1: add 4g p-nitroanisole (compound I), 0.26g cuprous chloride, 2.2g methoxyamine hydrochloride and 60mL DMF into a 100mL reaction flask, add 16g potassium tert-butoxide below 30°C The temperature was raised to 60-65 °C for 2 hours, and then cooled to room temperature; the reaction solution was poured into 150 mL of ice water, extracted 3 times with 50 mL of ethyl acetate, the organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 3.0 g of compound II crude product ; Add 600 mL of n-hexane to the crude compound II, heat under reflux for 1 hour, filter while hot, and dry the filter cake to obtain 2.1 g of pure compound II, yield: 47.8%. as attached Figures 1 to 2 Shown: the mass spectrum data of compound II is MS(ESI) m / z: 1...
Embodiment 2
[0032] N-(5-methoxy-2-phenoxyphenyl)methanesulfonamide (compound IV) is prepared by the preparation method of an Iguratimod intermediate of the present invention, and the specific operation is as follows:
[0033]Step S1: add 4g p-nitroanisole (compound I), 0.52g copper acetate, 6.6g methoxyamine hydrochloride and 60mL DMF to the 100mL reaction flask, add 7.7g sodium methoxide below 30°C; The reaction was carried out at 70-75 °C for 2 hours, and then cooled to room temperature; the reaction solution was poured into 150 mL of ice water, extracted three times with 50 mL of ethyl acetate, the organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 3.6 g of compound II crude product; 600 mL of n-hexane was added to the crude product II, heated to reflux for 1 hour, filtered while hot, and the filter cake was suctioned to dryness to obtain 2.6 g of pure compound II, yield: 59%. The mass spectrum data of pure compound II is MS(ESI) m / z: 169 (M+H)+...
Embodiment 3
[0037] N-(5-methoxy-2-phenoxyphenyl)methanesulfonamide (compound IV) is prepared by the preparation method of an Iguratimod intermediate of the present invention, and the specific operation is as follows:
[0038] Step S1: add 4g p-nitroanisole (compound I), 0.26g cuprous chloride, 3.32g methoxyamine salt and 60mL DMF to the 100mL reaction flask, add 9.7g sodium ethoxide below 30°C; The reaction was carried out at 75-80°C for 2 hours, and then cooled to room temperature; the reaction solution was poured into 150 mL of ice water, extracted three times with 50 mL of ethyl acetate, the organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 3.4 g of compound II crude product; 600 mL of n-hexane was added to the crude product II, heated under reflux for 1 hour, filtered while hot, and the filter cake was suction-dried to obtain 2.5 g of pure compound II, yield: 57%. The mass spectrum data of pure compound II is MS(ESI) m / z: 169 (M+H)+, which is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com