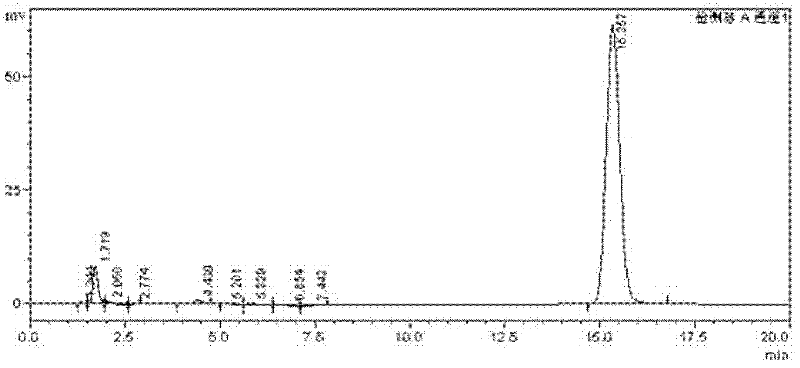
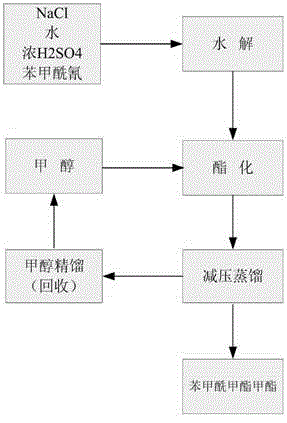
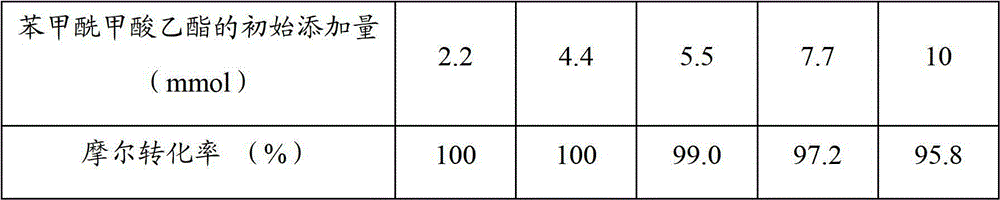
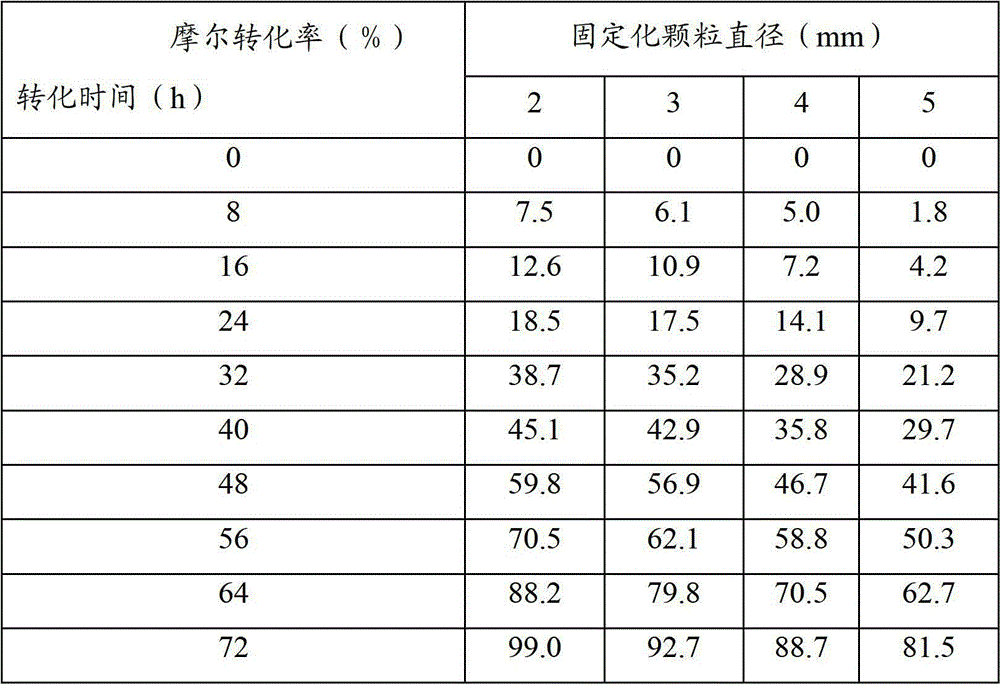
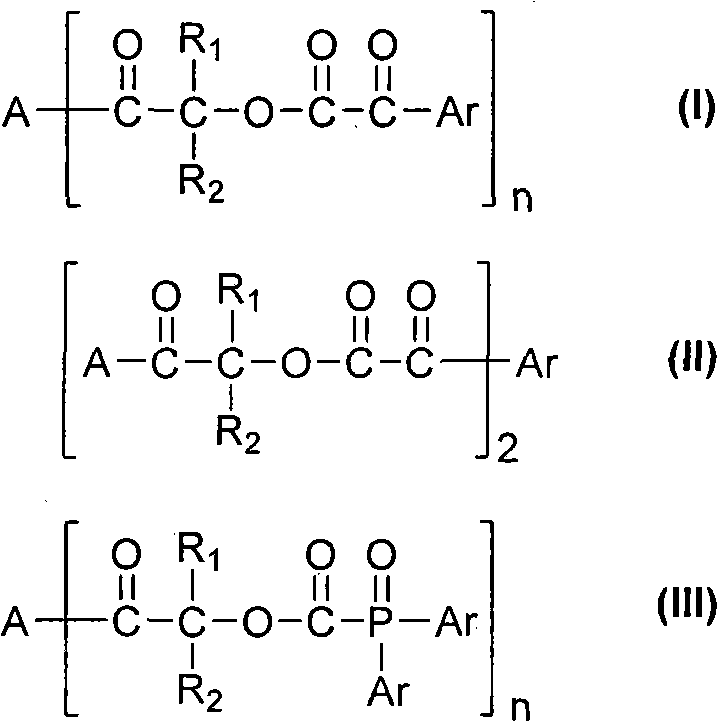Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
102 results about "Benzoylformic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
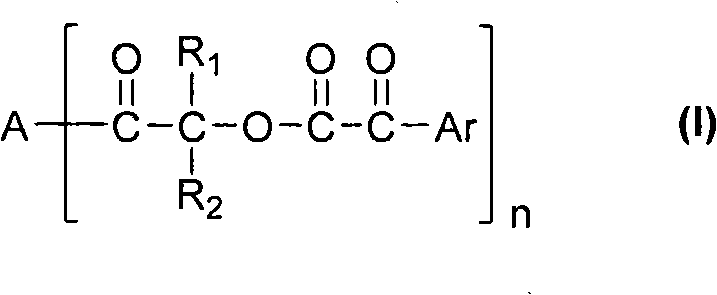
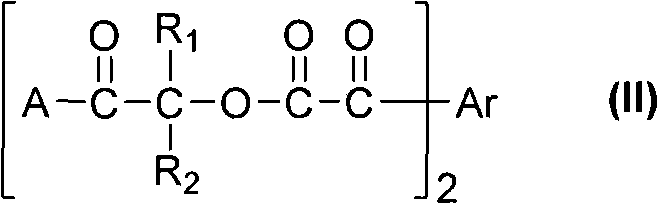
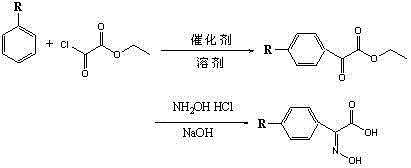
Multi-functionalized benzoylformicacid hydroxy-ketone ester compounds and photoinitiator containing compounds
The invention discloses novel Multi-functionalized benzoylformicacid hydroxy-ketone ester compounds, phosphorus-acyl derivatives, a combination containing the compounds, and a photoinitiator being used for photopolymerization of an alkene unsaturated compound system and containing the compounds. The compounds contain at least one of compounds shown in the following general formula I-III or any combination comprising the compounds shown in the following general formula I-III. The compounds can be used as alkene unsaturated compounds or the photoinitiator or photopolymerization of a mixt6ure containing the compounds, and can be combined with another photoinitiator and / or other additives for use.
Owner:SHENZHEN UV-CHEMTECH CO LTD
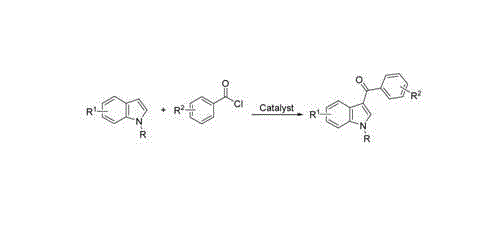
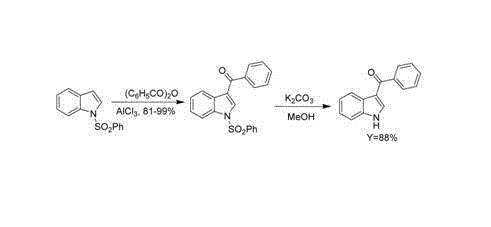
3-aroyl indole compound synthesis method
The invention discloses a synthesis method for 3 - aroyl indole compound with a formula I, which is characterized by comprising the following steps: taking R1-substituted indoles (II) and R2- substituted benzoylformic acid (III) as raw materials, copper salt as a catalyst, silver salt as an oxidant; and performing acylation reaction of decarboxylation in an organic solvent. According to the method, inexpensive and stable copper salt and silver salt are adopted; the reaction environment is not required to be subjected to absolute anhydrous processing; inert gas protection is not required, and the acylation reaction can be directly performed; and the reaction condition is mild and the yield is higher.
Owner:UNIV OF SCI & TECH LIAONING
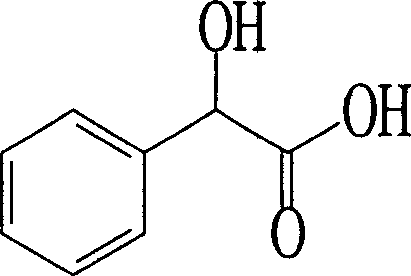
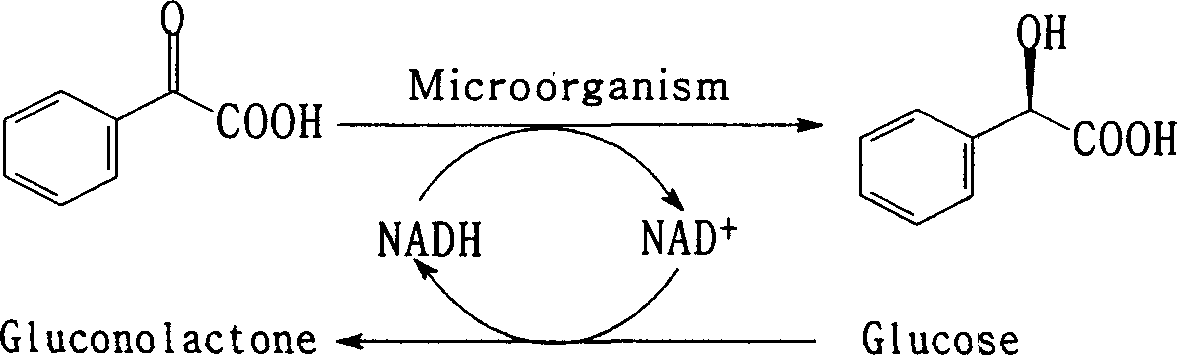
Method for producing optical homochiral amygdalic acid
This invention discloses a method for producing optical pure chiral mandelic acid. Use methyl benzoyl formate or the derivative of phenylglyoxylic acid as the bottom thing, the microorganism's cell of yeasts or mould whitlying etc. as catalyst, alternatively reduces the carbonyl in the bottom thing to the hydroxyl to get the chiral mandelic acid relying mainly on a kind of type (usually as R a type). This invention adopts chemistry-biology combination craft , with low cost, the chiral purity is high, easy to realize production for industrialization, it is easy to popularize and apply.
Owner:SHANDONG UNIV
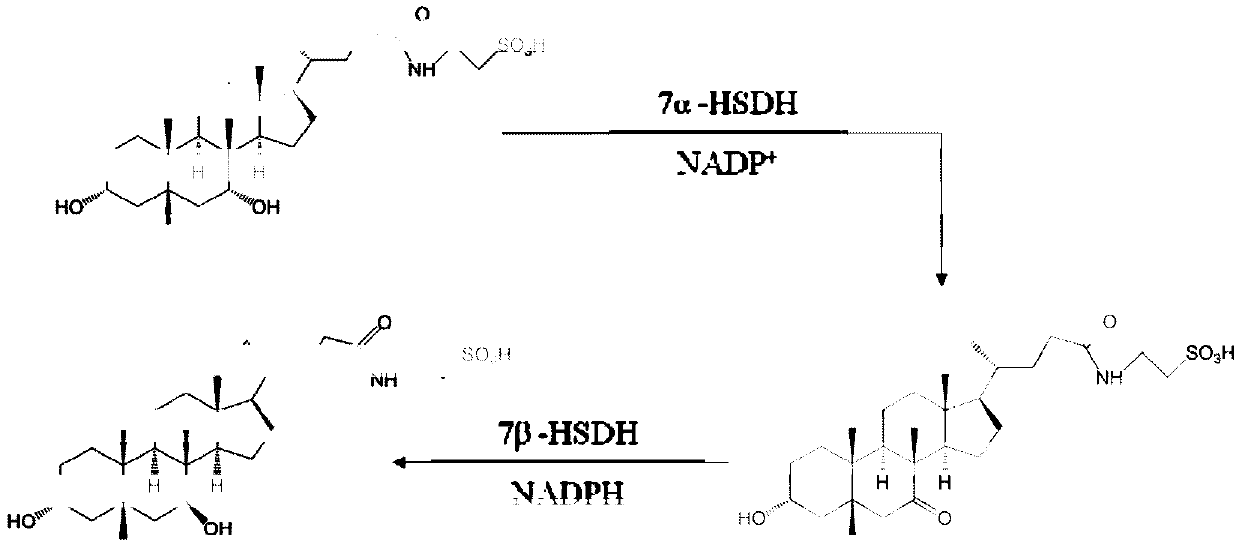
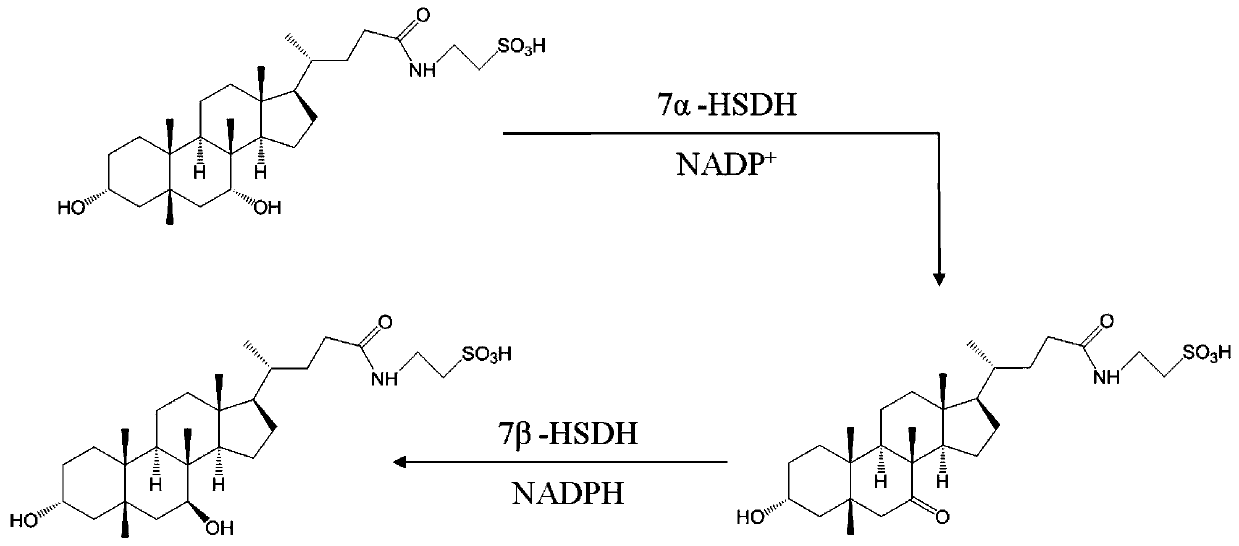
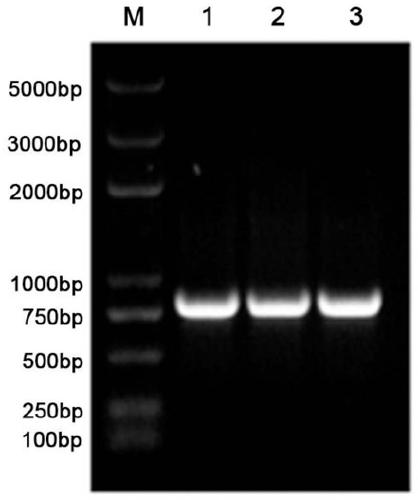
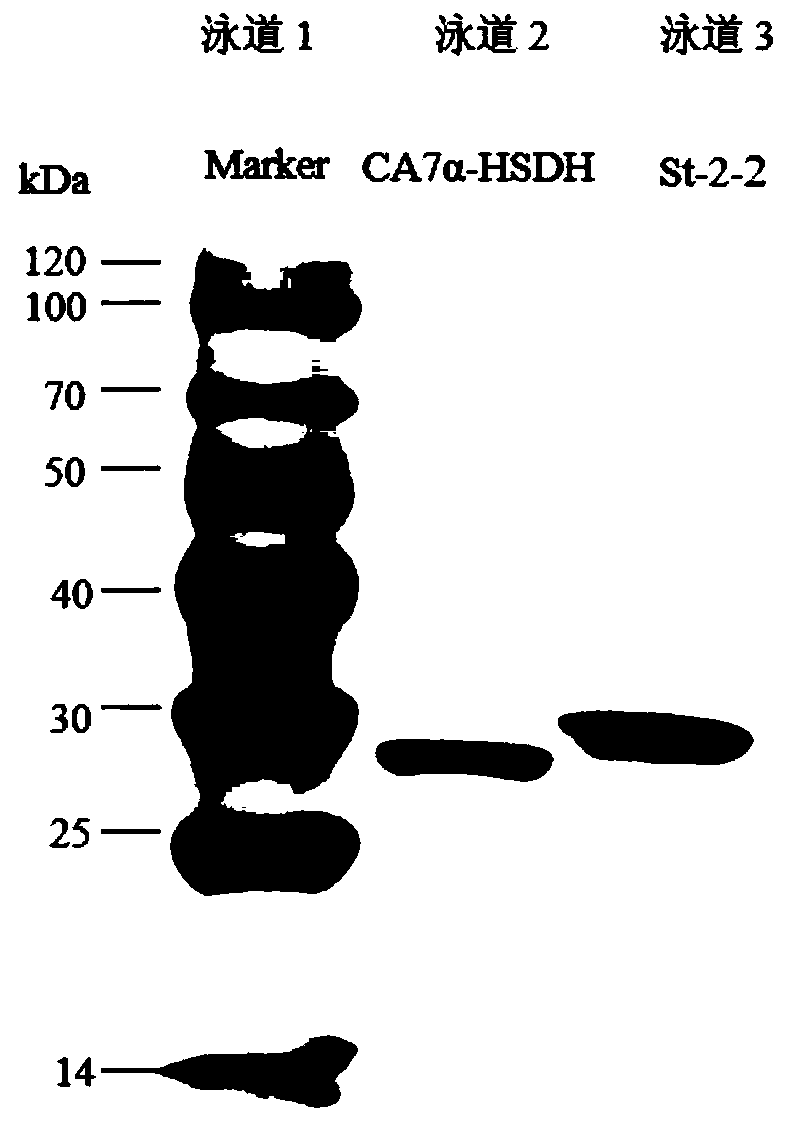
7 alpha-hydroxysteroid dehydrogenase as well as coding gene and application thereof
ActiveCN108034643ACatalytic asymmetric reduction reactionOxidoreductasesFermentationNucleotideKetone
The invention discloses 7 alpha-hydroxysteroid dehydrogenase as well as a coding gene and application thereof. An amino acid sequence of the 7 alpha-hydroxysteroid dehydrogenase provided by the invention is shown as SEQ ID NO. 1, and a nucleotide sequence of the 7 alpha-hydroxysteroid dehydrogenase gene is disclosed and shown as SEQ ID NO. 2. The 7 alpha-hydroxysteroid dehydrogenase can catalyze asubstrate taurocholic acid (TCA) to generate taurine 7-ketone cholic acid (T7K-CA), catalyze a substrate glycocholic acid (GCA) to generate glycine 7-ketone cholic acid (G7K-CA), catalyze a substratetaurochenodeoxycholic acid (TCDCA) to generate taurine 7-ketone lithocholic acid (T7K-LCA), catalyze a substrate glycochenodeoxycholic acid (GCDCA) to generate glycine 7-ketone lithocholic acid (G7K-LCA) and catalyze a substrate ethyl benzoylformate (EB) to generate ethyl 2-hydroxy-2-phenylacetate, and has better catalytic activity, has a catalytic activity to TCDCA, GCDCA and EB 10, 5, and 3 times that of sardinia clostridium 7 alpha-HSDH, correspondingly, and has great industrial application value.
Owner:CHONGQING UNIV
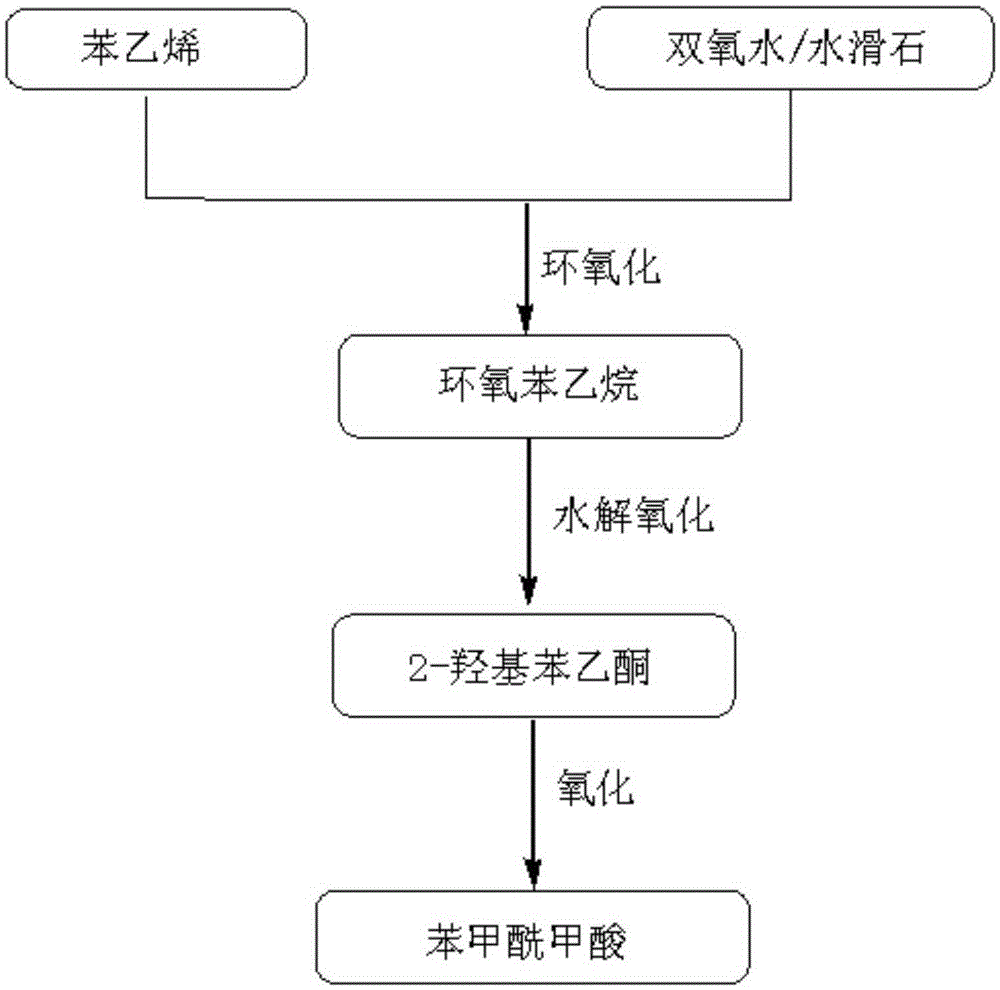
Benzoyl formic acid synthesis method
InactiveCN105330533AEasy to cleanHigh selectivityOrganic compound preparationCarboxylic compound preparationStyrene oxideSynthesis methods
The present invention discloses a benzoyl formic acid synthesis method comprising the following steps: styrene is used as a starting material for implementation of hydrogen peroxide catalytic epoxidation reaction to produce styrene oxide intermediate, alpha-Hydroxyphenylacetone is obtained by hydrolysis of styrene oxide, and the generated alpha-Hydroxyphenylacetone is further oxidized into the desired product benzoyl formic acid. Advantages of the method are that: by use of the method using a solid base for hydrogen peroxide catalytic oxidation of the styrene substrate to form a styrene oxide precursor material, the use of hydrobromic acid is avoided, industrial production reaction cleanability is improved, product preparation selectivity, overall yield and product purity are improved, reaction condition is mild, reaction time is short, the complexity of the reaction process is reduced and industrialization is easy.
Owner:CHINA PETROLEUM & CHEM CORP +1
Hydrocarbon oil demetalization agent and method for hydrocarbon oil demetalization
ActiveCN105733657AHigh removal rateWeak corrosiveRefining with non-metalsDewatering/demulsification with chemical means2-ketobutyric acidKeto acid
The invention relates to a hydrocarbon oil demetalization agent and a method for hydrocarbon oil demetalization. The hydrocarbon oil demetalization agent is a keto acid, wherein the keto acid is any of alpha-keto acid, beta-keto acid and gamma-keto acid, and the alpha-keto acid is one of pyruvic acid, 2-ketobutyric acid, phenylpyruvic acid and benzoyl formic acid; the beta-keto acid is acetoacetic acid; and the gamma-keto acid is 4-ketovaleric acid. The method comprises the steps of carrying out mixed contact on hydrocarbon oil, water, the keto acid, which serves as the demetalization agent, and a demulsifier, and then, carrying out electric desalting, so as to achieve oil-water separation and remove metals from the hydrocarbon oil. A molecule of the demetalization agent disclosed by the invention contains a difunctional group, can be in chelation with the metals and can also be in complexation with the metals, so that the demetalization agent has a high removal ratio to the metals in the hydrocarbon oil; according to the demetalization agent, the acidity is between the acidity of organic acids and the acidity of inorganic acids, the corrosiveness is lower than that of the inorganic acids, and the demetalization rate is higher than that of the organic acids; and meanwhile, the demetalization agent is free of elements such as phosphorus, sulfur and nitrogen and thus cannot cause adverse effects on subsequent processing.
Owner:PETROCHINA CO LTD +1
Clean production method of vanillin
ActiveCN104844436AAvoid decompositionInhibit aggregationOrganic compound preparationCarbonyl compound preparationSolventMandelic acid
The invention discloses a clean production method of oxidizing 3-methoxy-4-hydroxy mandelic acid for joint production of vanillin and superfine silver powder under an alkaline condition by adopting silver nitrate. The clean production method comprises the following steps: adding a silver nitrate solution, a 3-methoxy-4-hydroxy mandelic acid solution and a sodium hydroxide solution in a parallel flow manner into a reactor, reacting to generate a 3-methoxy-4-hydroxy acetophenone sodium solution and superfine silver powder, filtering and separating the generated silver powder, washing with deionized water and ethyl alcohol sequentially, and drying to obtain the superfine silver powder, wherein the reduction yield of the silver powder is 99.4 per cent to 100 per cent; acidizing filter liquor after the silver powder is separated, performing decarboxylation on 3-methoxy-4-hydroxy benzoylformic acid to generate the vanillin, using methylbenzene to extract the vanillin therein, recycling the methylbenzene solvent, and performing recrystallization on residues in an ethanol aqueous solution to obtain the vanillin, wherein the oxidation yield of the methoxy-4-hydroxy mandelic acid is 95.7 per cent to 98.5 per cent. The silver nitrate is taken as an oxidizing agent to prepare the vanillin, so that the oxidation yield is improved, and the oxidation reaction time is shortened. Two practical fine chemical products can be produced simultaneously, the raw materials can be sufficiently utilized, the generation of waste materials is reduced, the production cost is reduced, and the technological process is safe and environment-friendly.
Owner:TIANJIN VOCATIONAL INST
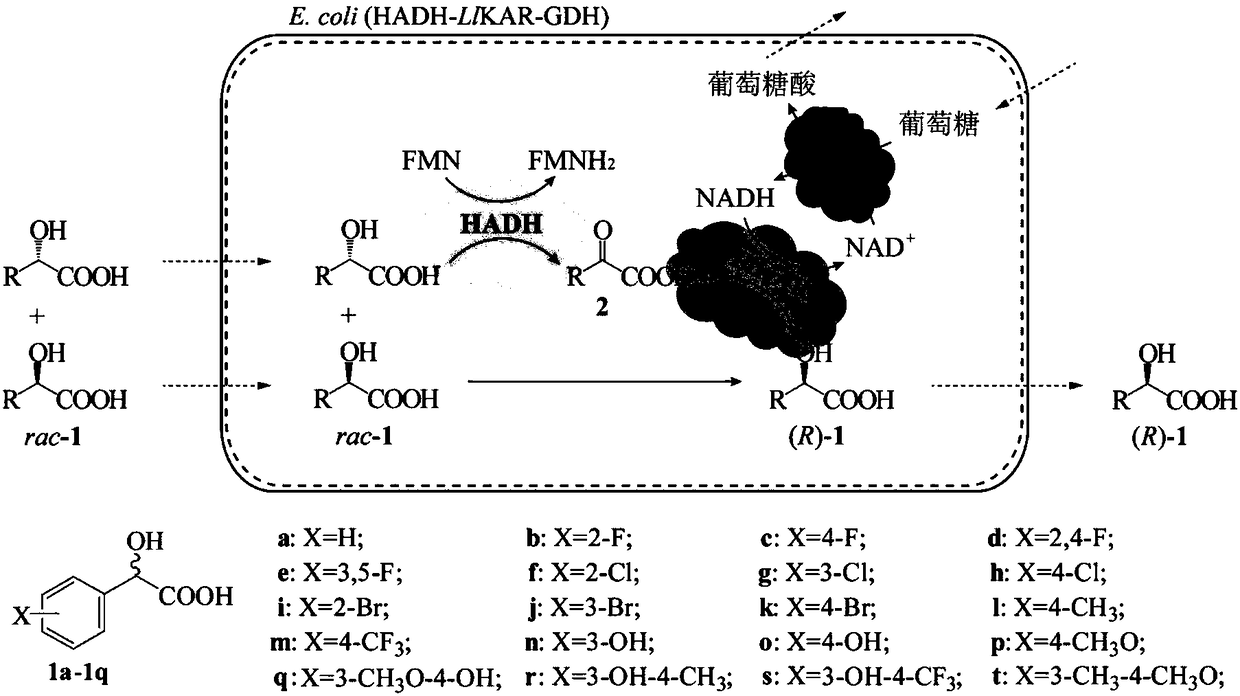
Keto acid reductase, gene, engineering bacterium and application of keto acid reductase in synthesis of chiral aromatic 2-hydroxy acid
ActiveCN108410831AHigh yieldImprove use valueBacteriaMicroorganism based processesEnzyme GeneLeuconostoc lactis
The invention discloses keto acid reductase, a gene, an engineering bacterium and application of the keto acid reductase in the synthesis of chiral aromatic 2-hydroxy acid. The keto acid reductase hasan amino acid sequence shown as in one of SEQ ID NO. 4, SEQ ID NO. 8 and SEQ ID NO. 10. The invention provides efficient keto acid reductase from Leuconostoc lactis; the efficient keto acid reductaseis capable of catalyzing broad-spectrum aromatic 2-keto acid, substrate loading capacity of a substrate of benzoylformic acid is increased from 100 mM to 400 mM. A single-bacterium dual-plasmid triple-enzyme series redox cascade system is established with the keto acid reductase, 2-hydroxy acid dehydrogenase and glucose dehydrogenase and is capable of catalyzing most racemic aromatic 2-hydroxy acid subjected to efficient deracemization to obtain chiral aromatic (R)-2-hydroxy acid, and both yield and e. e. value are greater than 99%; the system is finally applied to prepare optically pure (R)-2-chloromandelic acid by deracemization of 300 mM 2-chloromandelic acid, and the yield is up to 83.8 g / (L d).
Owner:ZHEJIANG UNIV OF TECH
Method for preparing high-strength hydrogel
The invention relates to the technical field of hydrogel and in particular relates to a method for preparing high-strength hydrogel. The method disclosed by the invention comprises the following steps: modifying the surface of montmorillonoid and polyvinyl alcohol by virtue of a silane coupling agent, forming a silanol group and carrying out a hydroxylation reaction; finally forming a polymolecular cover film on the surface of montmorillonoid and polyvinyl alcohol by the silane coupling agent, enabling phenol to enter the montmorillonoid and polyvinyl alcohol particle layer and carrying out apolymerization reaction, so that the interior of the hydrogel is effectively filled; performing cycle freezing and liquid nitrogen spray freezing for three times, so that the mechanical strength of the hydrogel is improved; and introducing 2-acrylamido-2-methyl propane-sulfonic acid, N,N-methylenebis acrylamide, methyl benzoylformate, gelatin, nano-fibers, modified quartz sand powder and deionizedwater mixture. By utilizing excellent mechanical properties of the gelatin, nano-fibers and modified quartz sand powder, the acting force is effectively dispersed, the mechanical strength of the hydrogel is further improved, and the high-strength hydrogel has wide application prospects.
Owner:戴琪
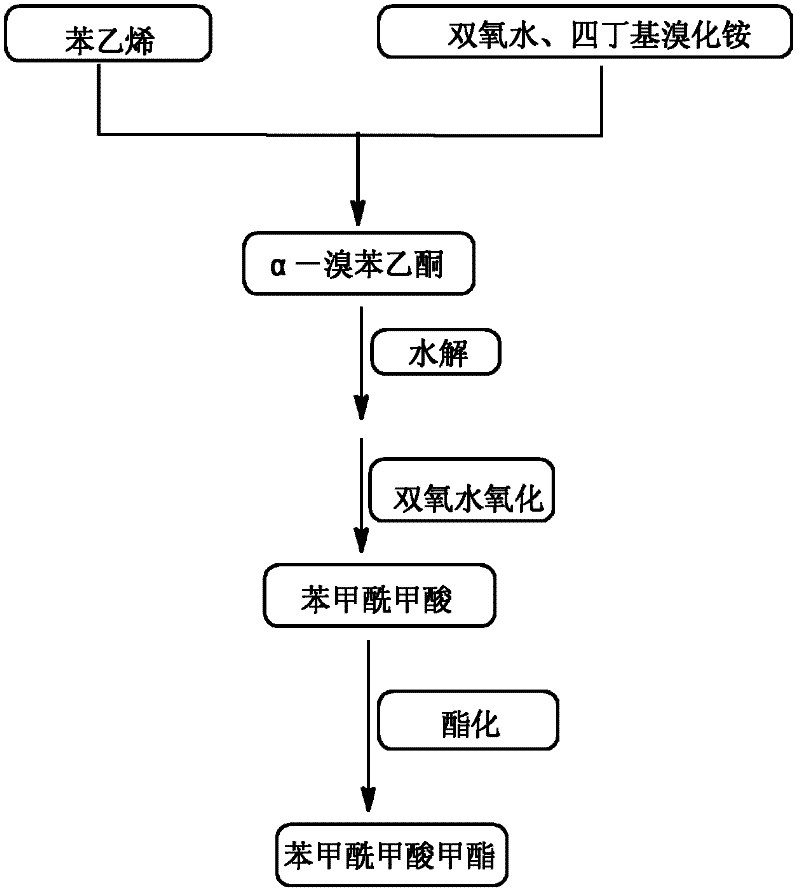
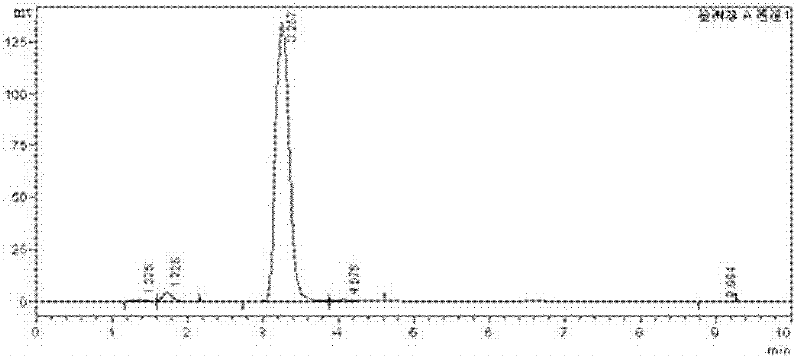
High-selectivity synthesis method of benzoyl formic acid
InactiveCN102336656AReduce generationEmission reductionOrganic compound preparationCarboxylic acid esters preparationSynthesis methodsAlpha-bromoacetophenone
The invention discloses a high-selectivity synthesis method of benzoyl formic acid, which comprises the following steps: oxidizing styrene, which serves as the raw material, in a H2O2 / tetrabutylammonium bromide mixed system to generate alpha-bromophenethyl alcohol; oxidizing the generated alpha-bromophenethyl alcohol with H2O2 to generate alpha-bromoacetophenone; hydrolyzing the alpha-bromoacetophenone under alkaline conditions to generate alpha-hydroxyacetophenone; and oxidizing the alpha-hydroxyacetophenone with H2O2 to generate the target product benzoyl formic acid. In the invention, oxydol is used as an oxidant, and the byproduct is water, thereby reducing the generation of the organic byproducts and the discharge amount of inorganic salt (acid) waste water. Compared with other inorganic heavy metallic salt and inorganic mineral acid oxidization methods, the method disclosed by the invention enhances the cleanness of industrial preparation reaction, and reduces the environmental pollution. The invention enhances the product yield and purity: the yield is enhanced by nearly 13%, the total yield is up to 93.7%, and the product purity exceeds 95%; and other indexes are correspondingly enhanced.
Owner:HEFEI UNIV OF TECH
Production method of metamitron
The invention discloses a production method of metamitron. The production method is characterized by comprising the following steps that benzoyl cyanide is hydrolyzed under the condition of H2SO4, and is then alcoholized into ester; hydrazine hydrate and methyl acetate take reaction to generate acetyl hydrazine hydrate; methyl benzoylformate and the acetyl hydrazine hydrate take reaction to generate hydrazine hydrate ester under the condition of H2SO4; hydrazine hydrate ester suspension and hydrazine hydrate take reaction; acyl is subjected to hydrazine treatment again to generate benzoyl hydrazine, and byproducts of methyl alcohol and water are generated; the benzoyl hydrazine is subjected to distillation, backflow, dehydration, cyclization and crystallization under the condition with a butanol solvent and a catalyst to generate the metamitron. The production method has the advantages that the cost of phase transfer catalysts is low; the cost of sodium benzoate is lower than that of sodium acetate, so that the production cost is reduced.
Owner:南通泰仓化学新材料有限公司
Efficient co-production strategy of L-phenylglycine and gluconic acid
ActiveCN106119272AGood market demandThe conversion process is fast and efficientOxidoreductasesFermentationEscherichia coliGluconic acid
The invention provides a method for co-producing L-phenylglycine and gluconate through single expression and co-expression of glucose dehydrogenase and L-leucine dehydrogenase in escherichia coli through utilizing a recombinant escherichia coli enzyme method and a whole cell method. The method is as follows: a glucose dehydrogenase gene and an L-leucine dehydrogenase gene are used for constructing recombinant single expression and co-expression carriers and are transformed into a gene engineering bacterium, namely the escherichia coli. The circulation of cofactors in a transformation system can be promoted through utilizing a recombinant bacterium enzyme method and the whole cell method; only a few of exogenous cofactors are added or the exogenous cofactors do not need to be used, and the L-phenylglycine and gluconic acid, which have high additional value, are co-produced by substrates including benzoylformic acid and glucose through utilizing a cofactor cyclic regeneration system; a transformation process is simple and rapid and low in cost. When transformation is carried out in a 5L fermentation tank for 2h to 4h, the yields of the L-phenylglycine and the gluconic acid, obtained by the method, can respectively reach 58.8g / L and 75.6g / L, and an actual and effective strategy is provided for industrial production.
Owner:JIANGNAN UNIV
Preparation method of (S)-(+)-ethyl mandelate by microbial transformed ethyl benzoylformate
InactiveCN102719496ACost-effective synthetic routeFacilitate reuseMicroorganism based processesOn/in organic carrierRecyclable catalystMicrobial transformation
The invention provides a preparation method of (S)-(+)-ethyl mandelate by using biocatalytic ethyl benzoylformate and taking saccharomyces cerevisiae with CGMCC No.2266 as a biocatalyst. The method comprises the following steps of: performing a conversion reaction for 8 to 120 hours at a temperature of between 20 and 35 DEG C in a water and n-hexane two-phase system by using ethyl benzoylformate as a substrate and the saccharomyces cerevisiae with CGMCC No.2266 as a biocatalyst; and separating and purifying the conversion solution to obtain (S)-(+)-ethyl mandelate. The microbial conversion method has the advantages of mild reaction condition, friendly environment, high product optical purity, high substrate conversion rate, simple separation and purification process and recyclable catalyst, and is suitable for industrial production.
Owner:杭州联豪科技有限公司
Method for synthesizing novel industrial copper extracting agent
InactiveCN102796026AExcellent extraction capacityExcellent separation of copper and ironOximes preparationPtru catalystHydroxylamine
The invention relates to a method for synthesizing a novel industrial copper extracting agent. The method comprises the following steps of: 1, performing synthesis by taking acylating agents of straight-chain alkylbenzene, ethyl chloroformylformate and the like as raw materials through acylation reaction under the catalytic action of lewis acid or a solid acid catalyst to form para-alkyl benzoyl ethyl formate (a); and 2, performing synthesis on the para-alkyl benzoyl ethyl formate (a) synthesized by the step 1 and hydroxylamine hydrochloride by hydrolysis reaction and oximation reaction under the alkaline condition to form carboxylic oxime (which is a copper extracting agent). Compared with copper extracting agents which are used industrially at present, the industrial copper extracting agent synthesized by the method has the advantages that the industrial copper extracting agent can be used under the high-acidity condition, the extraction capacity, the degree of separation of copper and iron and the reextraction performance of the industrial copper extracting agent are superior or equal to the copper extracting agents, and the production cost of the industrial copper extracting agent is lower than that of hydroxyl oxime extracting agents. In addition, by the synthetic process, the requirements of energy conservation, consumption reduction and environment friendliness also can be met.
Owner:JIANGXI UNIV OF SCI & TECH
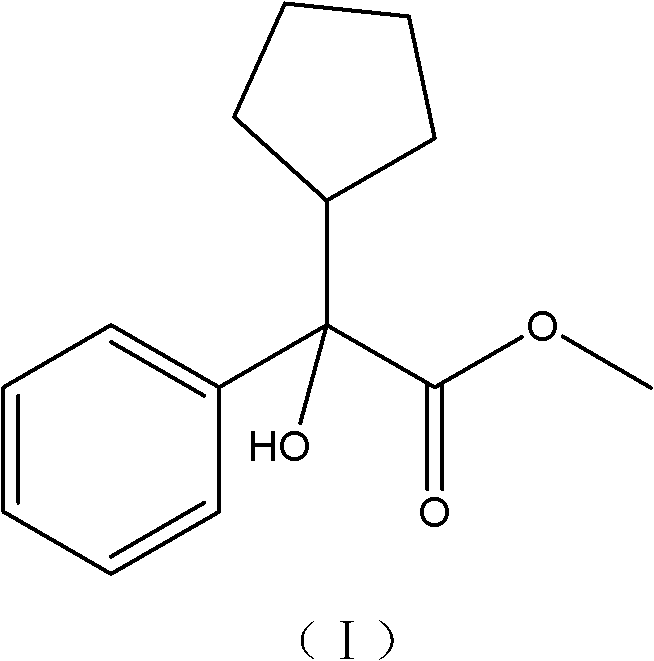
Preparation method of a-cyclopentyl methyl mandelate
InactiveCN102718654AWide variety of sourcesEasy accessOrganic compound preparationCarboxylic acid esters preparationGrignard reagentHydrolysis
The invention discloses a preparation method of a-cyclopentyl methyl mandelate, comprising the following steps of: (1) Grignard reagent preparation of a-cyclopentyl chloride: performing a reaction between magnesium granules and cyclopentyl chloride in an ether or tetrahydrofuran solvent to generate an a-cyclopentyl chloride Grignard reagent; (2) preparation of an a-cyclopentyl methyl mandelate crude product: firstly dissolving benzoyl methyl formate in the ether or tetrahydrofuran solvent to form a benzoyl methyl formate solution, and performing a reaction between the a-cyclopentyl chloride Grignard reagent generated during the step (1) and the benzoyl methyl formate solution to generate the a-cyclopentyl methyl mandelate crude product; and (3) purification of the a-cyclopentyl methyl mandelate crude product: carrying out hydrolysis and esterification on the a-cyclopentyl methyl mandelate crude product obtained from the step (2) to obtain the pure a-cyclopentyl methyl mandelate. The raw materials of benzoyl methyl formate, magnesium granules and cyclopentyl chloride are all commonly-used reagents, have wide sources and are easy to obtain. In addition, the preparation method has advantages of simple steps for synthesis, low cost, low toxicity, convenient operation and high security.
Owner:BEIJING HEZHONGMING MEDICAL TECH
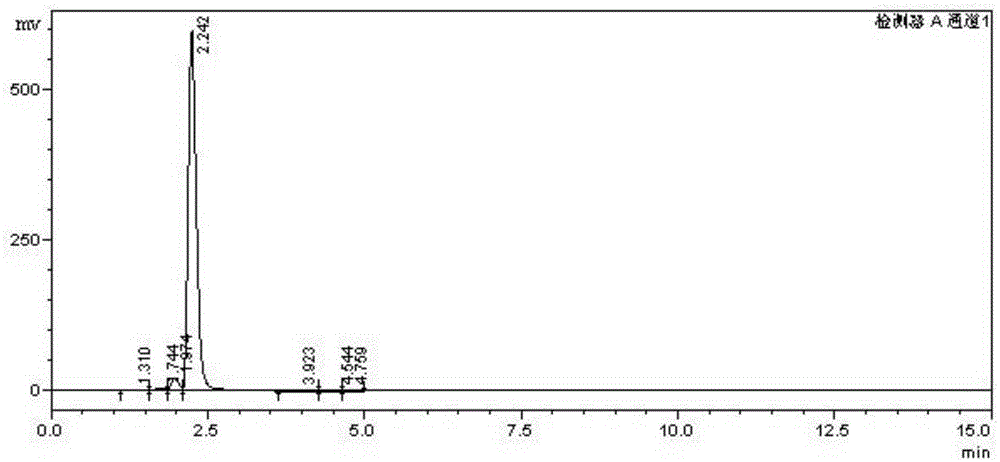
Process for preparing (R)-mandelic acid by microbial asymmetric reduction
InactiveCN1840670AGood conversion effectSimple compositionFungiMicroorganism based processesMicroorganismSynthesis methods
The asymmetrical reduction bio-method for (R)-mandelic acid comprises: screening the Saccharomyces cerevisiae AS 2.150 for culture and full cell preparation; with benzoylformic aicd as substrate, adding assist substrate, catalytic converting to obtain the product with optical purity up to 90%e.e. This invention improves product optical purity and yield, and has important meaning for enzyme development and resolution.
Owner:JIANGNAN UNIV
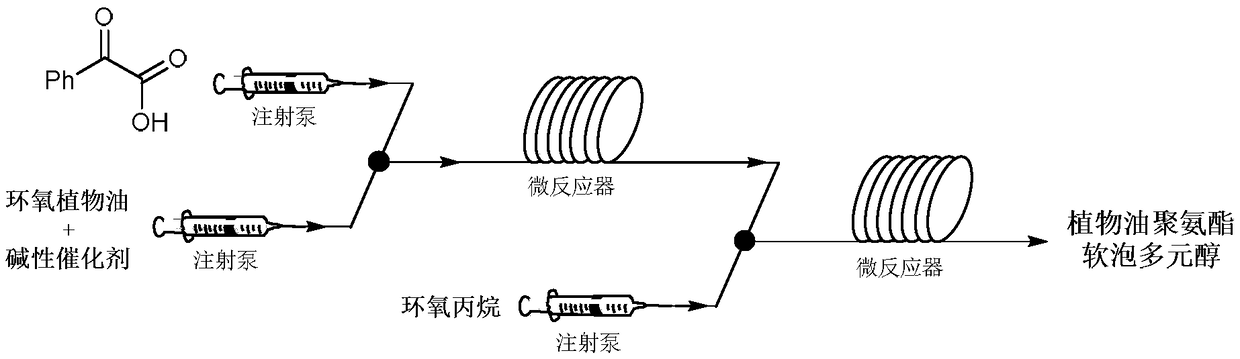
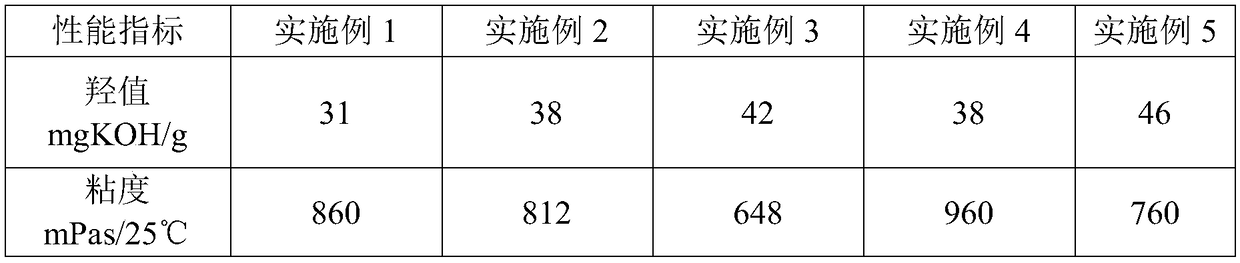
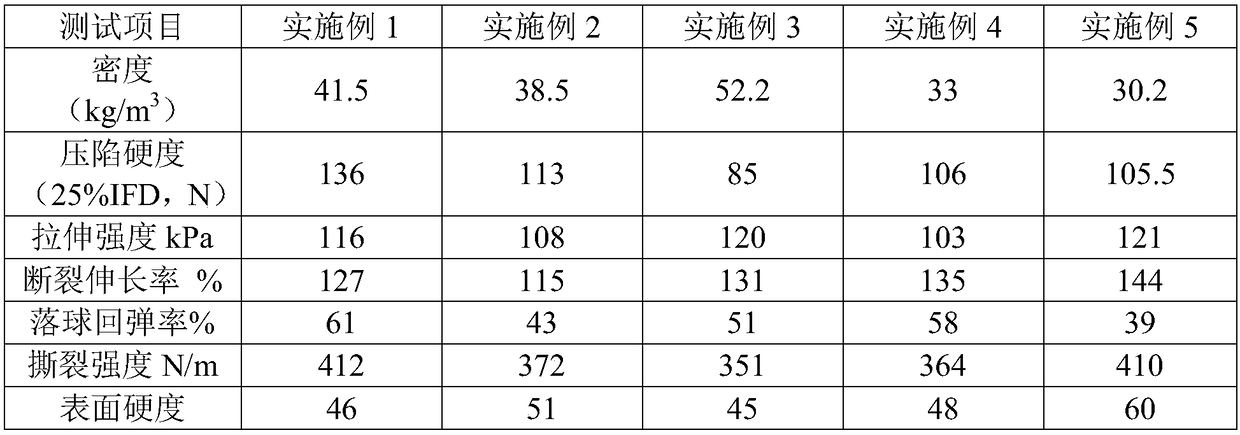
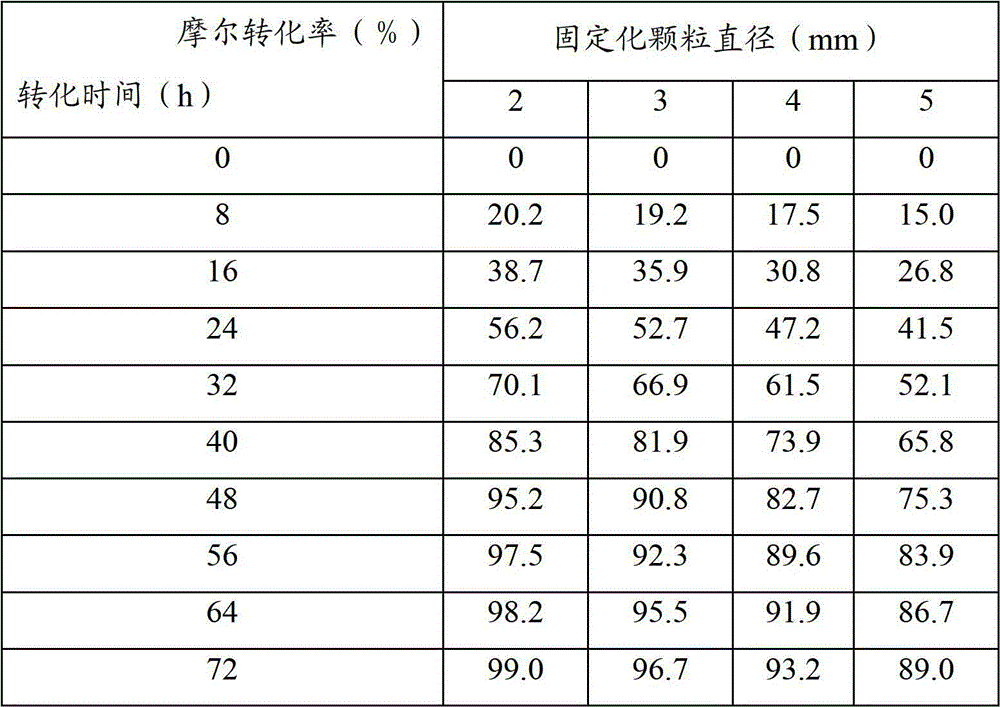
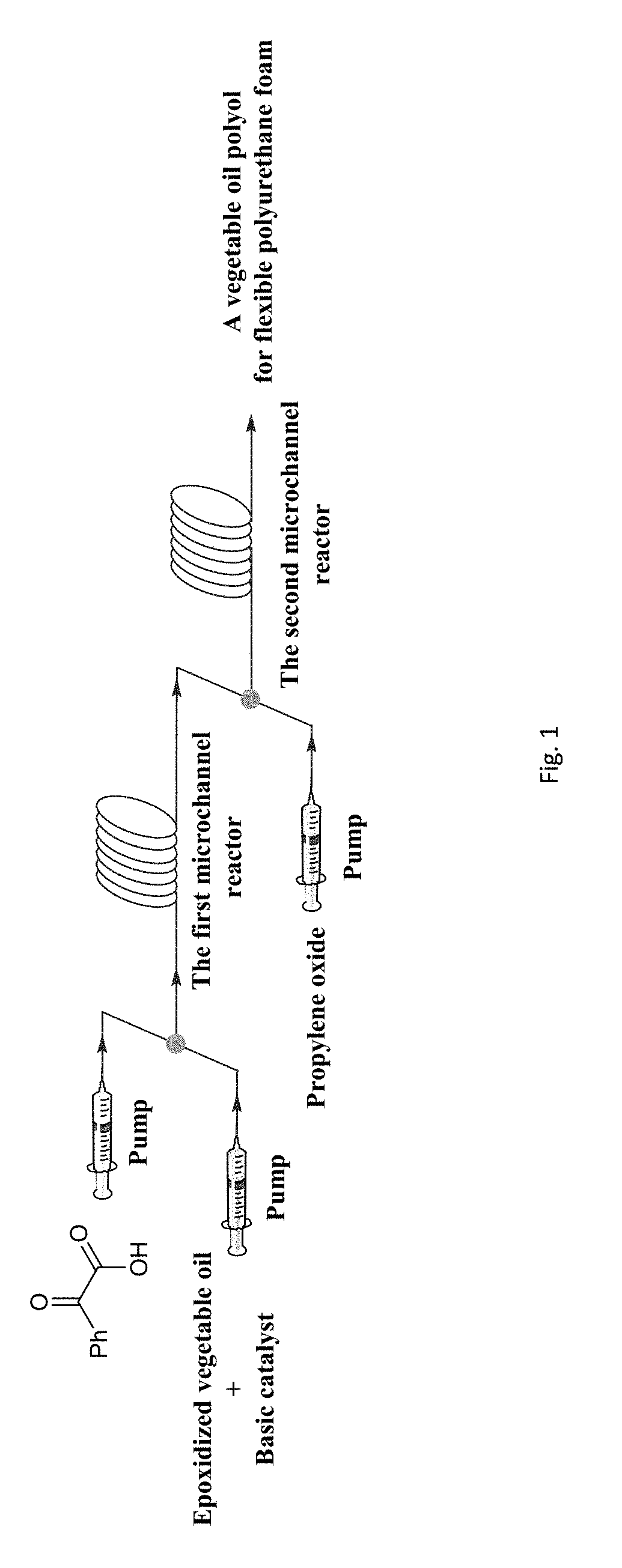
Vegetable oil polyurethane soft foam polyol, and preparation method and application thereof
The invention discloses vegetable oil polyurethane soft foam polyol, and a preparation method and an application thereof. The method comprises the following steps: (1) carrying out ring-opening reaction on epoxy vegetable oil, benzoyl formic acid, a basic catalyst and an inert solvent in a first microchannel reactor of a microchannel reaction device to obtain vegetable oil polyol; and (2) carryingout addition polymerization reaction on the vegetable oil polyol obtained in the step (1), epoxy propane and an inert solvent in a second microchannel reactor of the microchannel reaction device to obtain the vegetable oil polyurethane soft foam polyol. The obtained vegetable oil polyurethane soft foam polyol is novel in structure and can completely replace the traditional petrochemical polyol tobe applied to the preparation of polyurethane foam materials.
Owner:NANJING TECH UNIV
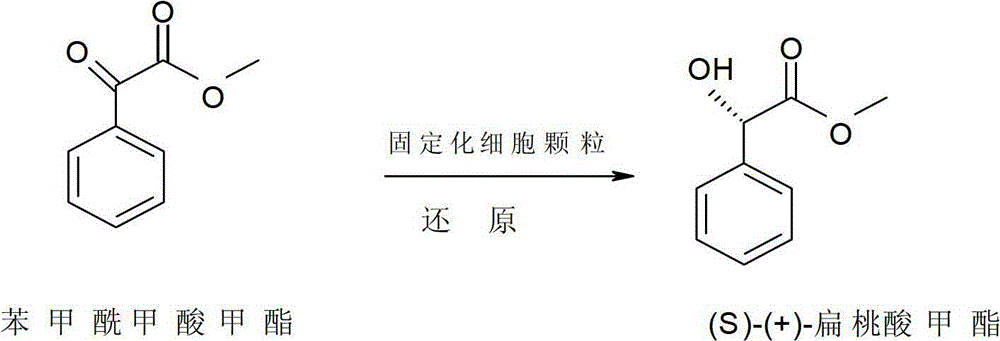
Method for preparing methyl (S)-(+)-mandelate by microbial transformation of methyl benzoylformate
ActiveCN102719497ALow costEasy to achieve separation and extractionMicroorganism based processesChemical recyclingMicroorganismMicrobial transformation
The invention provides a method for preparing methyl (S)-(+)-mandelate by the microbial transformation of methyl benzoylformate. The method comprises the following steps of: performing transformation reaction at the temperature of 20 to 35 DEG C for 8 to 96 hours in dibutyl phthalate by taking methyl benzoylformate as a substrate, and enzyme-containing thallus cells obtained by fermenting a Saccharomyces cerevisiae strain CGMCC No.2230 as a biocatalyst, and separating and purifying a transformation solution to obtain a product, namely the methyl (S)-(+)-mandelate. Reaction conditions are mild, the microbial transformation method is environment-friendly and is suitable for industrialized production, the product has high optical purity, the substrate is high in transformation rate, the separation and purification process is simple, and the catalyst can be recycled.
Owner:ZHEJIANG UNIV OF TECH
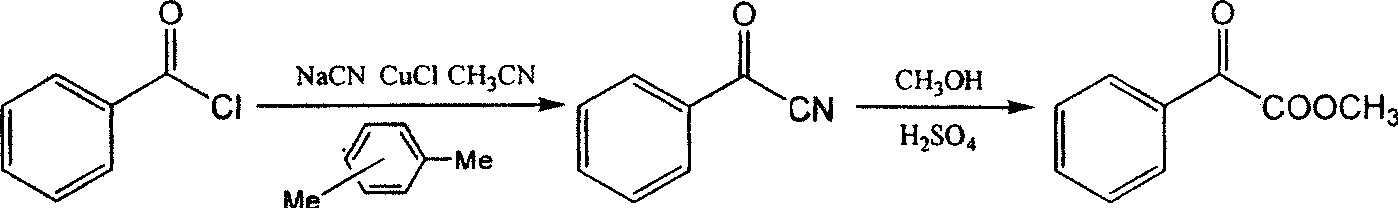
Novel process for synthesizing methyl benzoylformate from benzoyl cyanide
InactiveCN107473971ASolve the problem that low temperature curing affects the reactionThe reaction speed at low temperature is acceleratedOrganic compound preparationCarboxylic acid esters preparationOrganic solventCyanide
The invention discloses a novel process for synthesizing methyl benzoylformate from benzoyl cyanide. The novel process comprises the following steps: dissolving benzoyl cyanide in an organic solvent, adding a halide catalyst and a phase transfer catalyst, lasting for 5-60 minutes at a temperature of 5-20 DEG C, adding dropwise concentrated sulfuric acid to the system, and carrying out thermal reaction for 0.5-5 hours at a temperature of 20-40 DEG C to obtain an intermediate iminosulfate compound; and lasting for 5-60 minutes at a temperature of 30-50 DEG C, adding dropwise methyl alcohol to the system, and carrying out thermal reaction for 0.5-5 hours at a temperature of 50-100 DEG C to obtain methyl benzoylformate. In the novel process disclosed by the invention, the reaction at a low temperature is accelerated obviously by using suitable solvents and composite catalysts, so that the potential safety hazard in industrialized production is eliminated, the stability of production is improved, the energy consumption is reduced, and the yield is up to 96%.
Owner:安道麦股份有限公司
Durable floor paint
InactiveCN104356900AImprove wear resistanceWith strengthPolyester coatingsPolymer science(Hydroxyethyl)methacrylate
The invention discloses durable floor paint which comprises the following raw materials in parts by weight: 9 to 14 parts of alkyd resin, 4 to 6 parts of an antifoaming agent, 5 to 11 parts of wear-resistant auxiliary, 3 to 9 parts of organic bentonite, 6 to 14 parts of hydroxyethyl methacrylate, 2 to 8 parts of basic lead carbonate, 3 to 6 parts of montmorillonite, 4 to 8 parts of methyl benzoylformate, 3 to 5 parts of butyl acetate, 3 to 7 parts of precipitated silica, 5 to 10 parts of hydrogenated castor oil, 1 to 3 parts of ash calcium powder, 4 to 6 parts of lacquer wax and 4 to 7 parts of amine salt dispersant. The durable floor paint has the benefits of high wear resistance, certain strength and high brightness.
Owner:QINGDAO KELIKE INFORMATION TECH
Vegetable Oil Polyol for Flexible Polyurethane Foam and Preparation Method and Application Thereof
A vegetable oil polyol for flexible polyurethane foam, a preparation method and application thereof. The method includes the following steps: (1) subjecting an epoxidized vegetable oil, a benzoylformic acid, a basic catalyst, and an inert solvent to a ring-opening reaction in a first microchannel reactor of a microchannel reaction device to obtain a vegetable oil polyol; and (2) subjecting the vegetable oil polyol obtained in the step (1), a propylene oxide and an inert solvent to an addition polymerization reaction in a second microchannel reactor of the microchannel reaction device to obtain the vegetable oil polyol for flexible polyurethane foam.
Owner:NANJING UNIV OF TECH
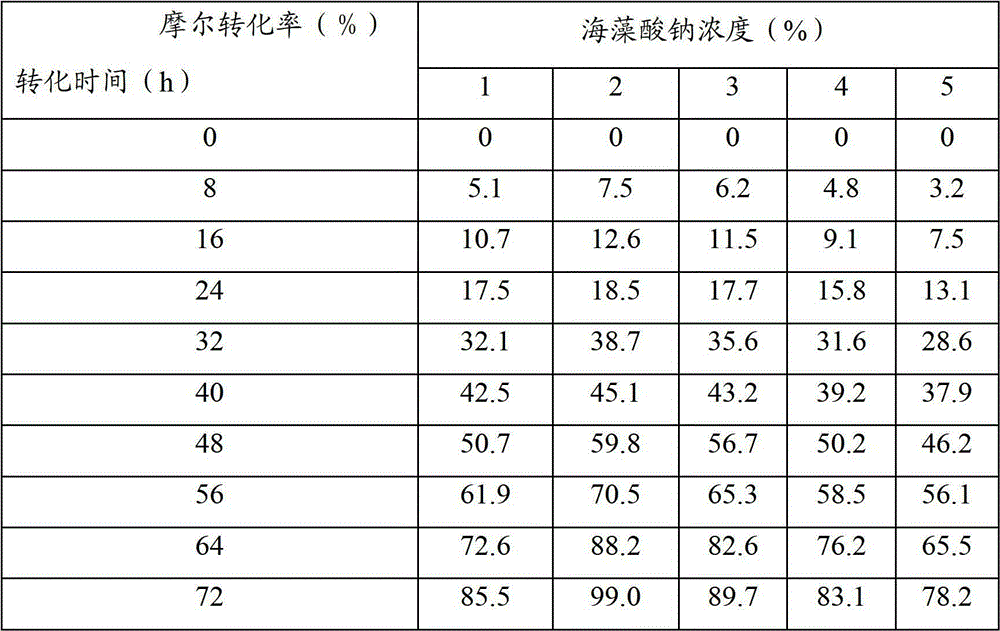
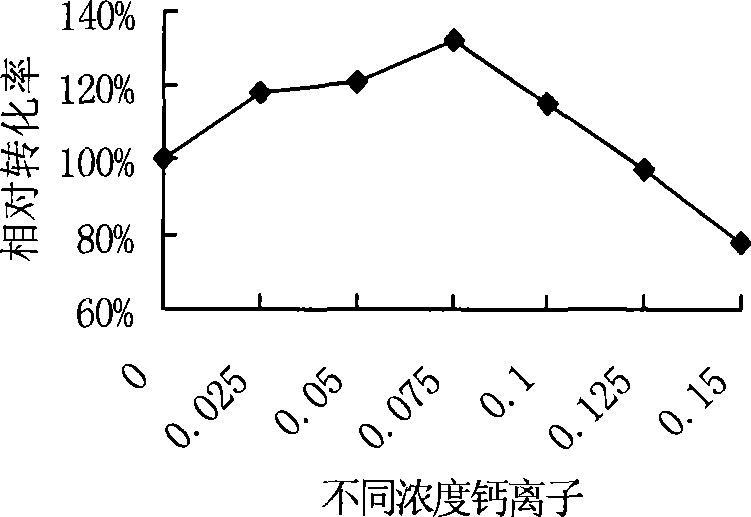
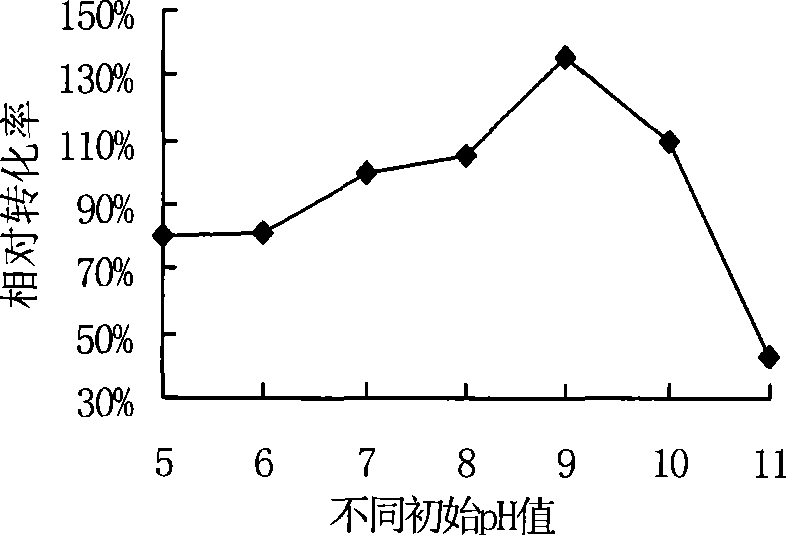
Culture medium for producing benzoylformic acid by fermentation method
InactiveCN101463369AIncrease the amount of feedHigh reaction specificityFermentationBenzoylformic acidFermentation
The invention discloses a culture medium for producing benzoylformic acid by fermentation method. The culture medium comprises calcium ion and D, L-phenylglycine and is characterized in that the initial pH value of the culture medium ranges from pH more than 7 to pH equal to 10. By adopting the culture medium to produce the benzoylformic acid, the substrate inventory rating can increase to 50g / L; the conversion rate can reach 36.8%, the response-specific is high, almost no byproduct exists, simple extraction is enough, and the operation is very convenient.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
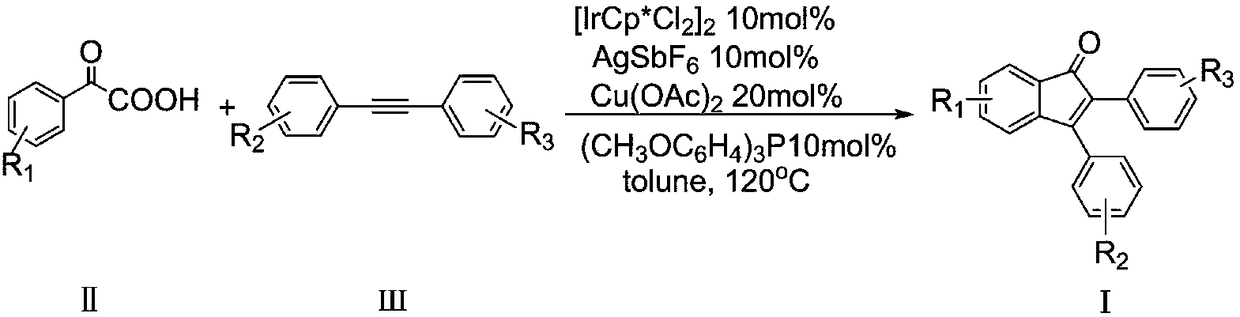
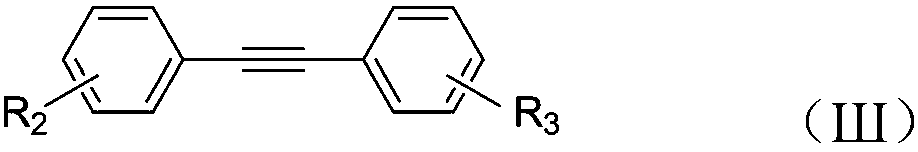
Synthesis method for 2,3-diphenyl-1H-indene-1-one derivatives
InactiveCN109180446AImprove conversion efficiencyGood atom economyOrganic compound preparationCarboxylic acid esters preparationIridiumPentamethylcyclopentadiene
The invention discloses a synthesis method for 2,3-diphenyl-1H-indene-1-one derivatives. The synthesis method comprises the steps of: adding benzoyl formic acid, a diphenyl acetylene compound, pentamethyl cyclopentadiene dichloride iridium, copper acetate, silver hexafluoroantimonate and a silver hexafluoroantimonate into an organic solvent; heating for reaction in the presence of air; and after the reaction, carrying out post-treatment to obtain the 2,3-diphenyl-1H-indene-1-one derivative. The method synthesizes the 2,3-diphenyl-1H-indene-1-one derivative in one step through simple and easilyavailable raw materials, so that the conversion efficiency is high and the atomic economical benefit is good. Meanwhile, the synthetic method is simple to operate, high in reaction yield and wide inprimer adaptability.
Owner:JILIN INST OF CHEM TECH
7alpha-oxhydryl sterol dehydrogenase and encoding gene and application thereof
The invention relates to 7alpha-oxhydryl sterol dehydrogenase and an encoding gene and application thereof, and belongs to the technical field of biology. The 7alpha-oxhydryl sterol dehydrogenase is protein shown in SEQ ID NO.1 or protein with the same functions, obtained through substitution and / or deletion and / or addition of one or more amino acid residues of an amino acid sequence shown in theSEQ ID NO.1. The 7alpha-oxhydryl sterol dehydrogenase can catalyze an asymmetric reduction reaction of carbanyl groups of C7alpha-oxhydryl groups of taurocholic acid, glycocholic acid, taurochenodeoxycholic acid and glycochenodeoxycholic acid and catalyze an asymmetric reduction reaction of carbanyl groups and oxhydryl groups in benzoyl groups of ethyl benzoylformate. Compared with 7alpha-oxhydrylsterol dehydrogenase in clostridium sardiniense, the 7alpha-oxhydryl sterol dehydrogenase has higher catalytic activity and thermostability, and has very high industrial application value.
Owner:CHONGQING UNIV
Low-yellowing material fading prevention UV primer and preparation method thereof
InactiveCN112940605AGood yellowing resistanceImprove heat resistancePolyurea/polyurethane coatingsPriming paintsMeth-Ultraviolet lights
The invention relates to a low-yellowing material fading prevention UV primer and a preparation method thereof. The UV primer comprises, by weight, hexafunctional aliphatic polyurethane acrylate, organic silicon modified trifunctional aliphatic polyurethane acrylate, bifunctional aliphatic polyurethane acrylate, ethoxylated trimethylolpropane triacrylate, 1, 6-hexanediol diacrylate, a photoinitiator, talcum powder, an ultraviolet light absorber, an antioxidant, a defoaming agent, a dispersing agent and an anti-settling agent according to specific parts. The photoinitiator comprises methyl benzoylformate and 2, 4, 6-trimethylbenzoyl-diphenyl phosphine oxide according to the mass ratio of 1: (0.1-0.5); and the ultraviolet light absorber is composed of a benzotriazole ultraviolet light absorber and a hindered amine ultraviolet light absorber according to the mass ratio of 1: (0.3-0.8). The UV primer has low yellowing, can prevent color fading of dyed wood and has excellent adhesive force, and the defects that the dyed wood is easy to change color and fall off in the production and use processes can be effectively avoided.
Owner:东莞大宝化工制品有限公司 +2
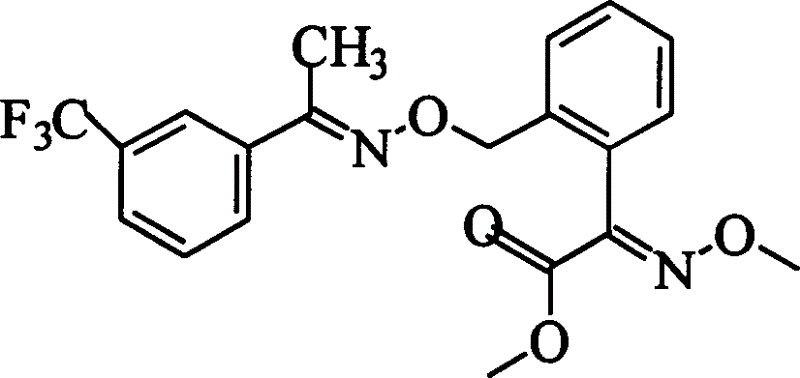
Liquid phase synthesis process of oxime strain ester by poly-ethandiol
InactiveCN1560028ASimplify separation and purification stepsBroad mindOximes preparationPolyethylene glycolAcetophenone
The invention discloses a method of synthesizing trifloxystrobin by using liquid phase polyglycol: (a) with the help of hydroxyl on the olyglycol to make condensation reation with o-methyl benzoylformic acid, loading the o-methyl benzoylformic acid on the olyglycol, so as to obtain 2-(2'-methylphenyl)-2- polyglycol carboxy acetate; (b) making the product obtained in step (a) react with methoxy amine and acid salts, obtaining 2-(2'-methylphenyl)-2- polyglycol carboxy acetate-O-methyl ketone oxime; (c) bromizing the product obtained in step (b) to obtain 2-(2'-bromomethylphenyl)-2- polyglycol carboxy acetate-O-methyl ketone oxime; (d) on basic condition, condensing the compound obtained in step (c) with meta-trifluoromethyl acetophenone oxime to obtain 2-[1'-{[(3'-trifluoromethylphenyl)-ethyl-imine]O}-O-tolyl]-2-polyglycol carboxy acetate-O- methyl ketone oxime; (e) making ester exchange reaction of the compound obtained in step (d) with methanol to obain the trifloxystrobin. On the basis of not changing properties of trifloxystrobin, it simplifies the step of separating and purifying in reacting course, reducing the cost.
Owner:TONGJI UNIV
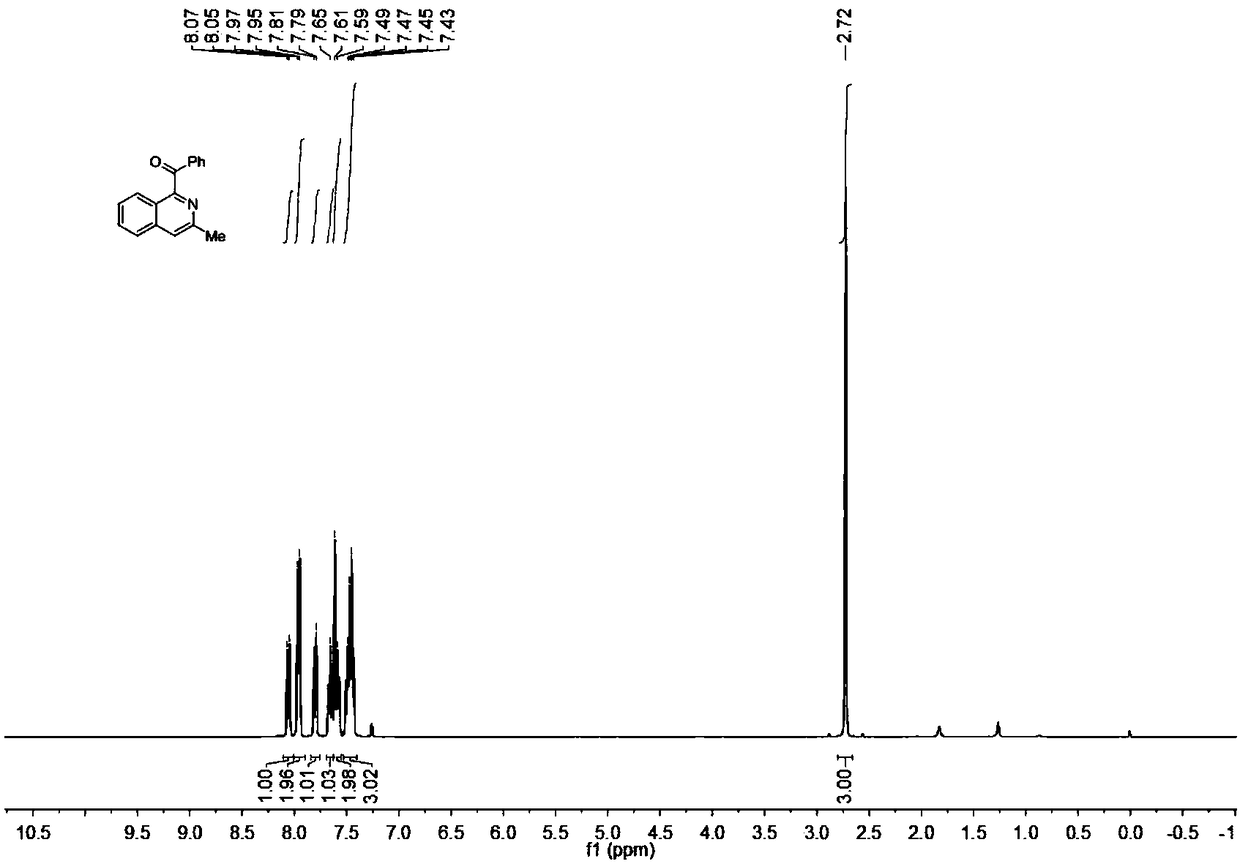
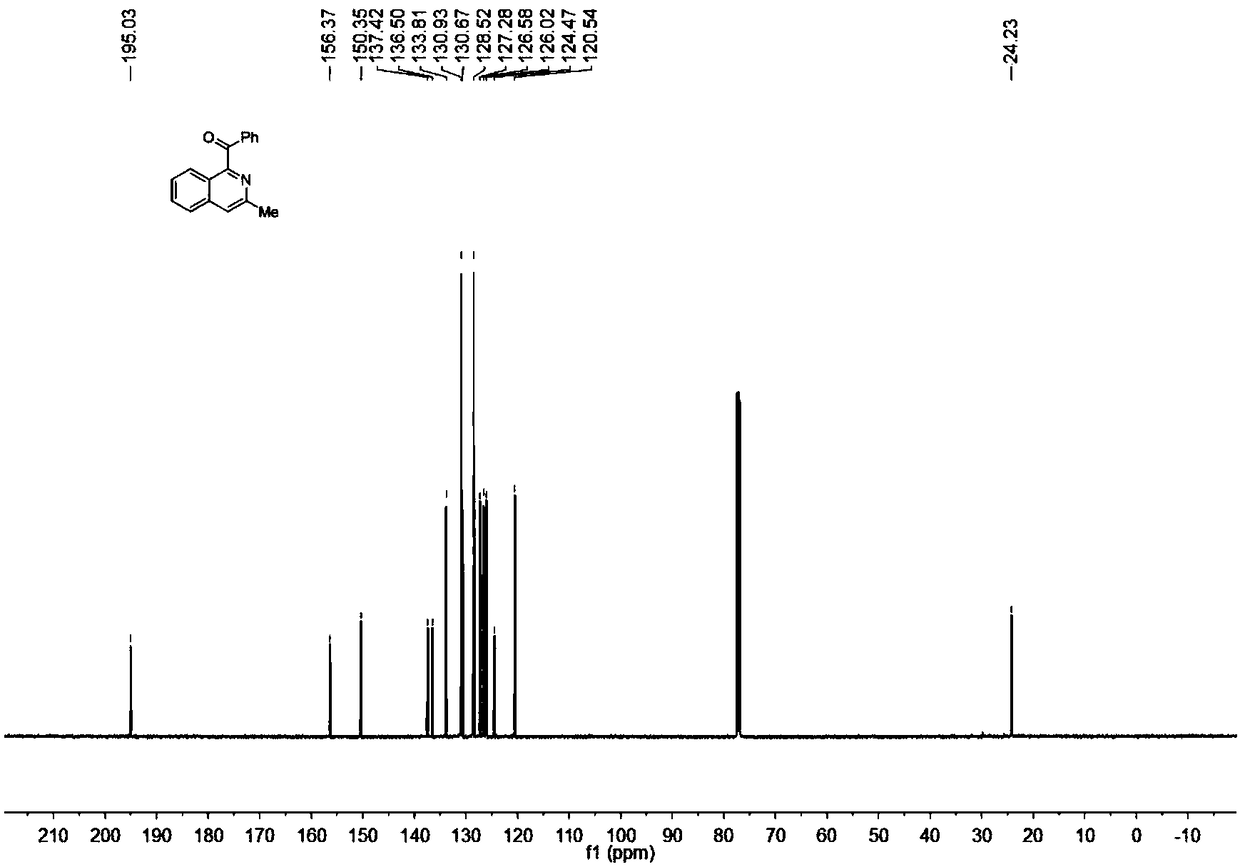
Synthesis method of acylated quinoline or isoquinoline derivative
ActiveCN108299291AThe order of addition is arbitrarySimple reaction conditionsOrganic chemistryIridiumOrganic synthesis
The invention discloses a synthesis method of an acylated quinoline or isoquinoline derivative, belongs to the field of organic synthesis, and aims to solve the problems of complicated reaction, severe conditions and low environmental friendliness in the conventional acylated quinoline or isoquinoline derivative synthesis method. The synthesis method comprises the following steps: under a nitrogenatmosphere, adding quinoline or isoquinoline and benzoyl formic acid which are used as reaction raw materials, persulfate used as an oxidant and a metal iridium compound used as a visible light catalyst into a reaction container together with an organic solvent for reaction under a visible light illumination condition, and at the end of the reaction, filtering, concentrating, separating and purifying a reaction system, thus obtaining the acylated quinoline or isoquinoline derivative. According to the synthesis method, under the nitrogen atmosphere, the reaction system is placed under a visible light source for illumination to realize reaction, so that the whole reaction process is safe and environmentally friendly, reaction conditions are simple, and operation steps are convenient; the synthesis method belongs to an environment-friendly chemical process.
Owner:杭州量创科技咨询有限公司
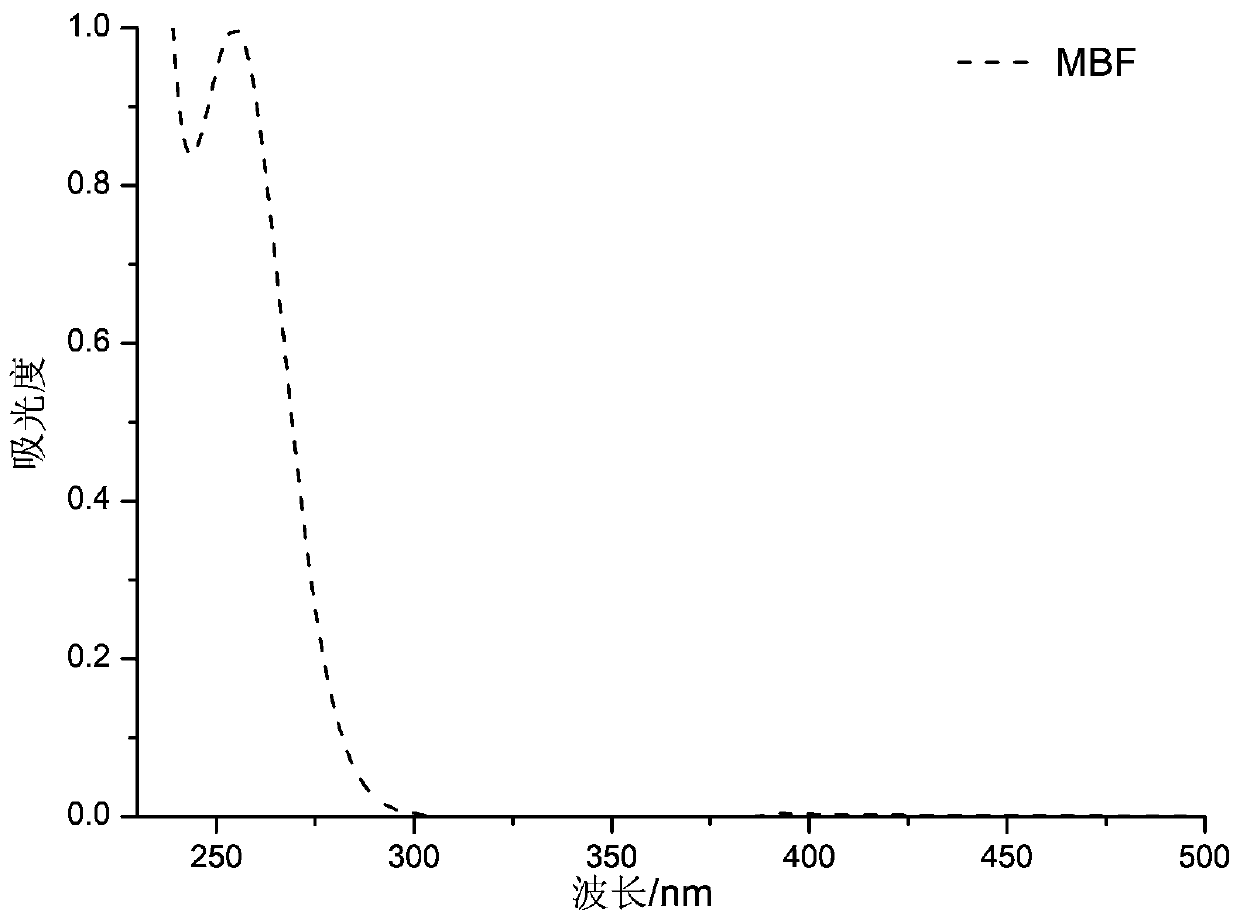
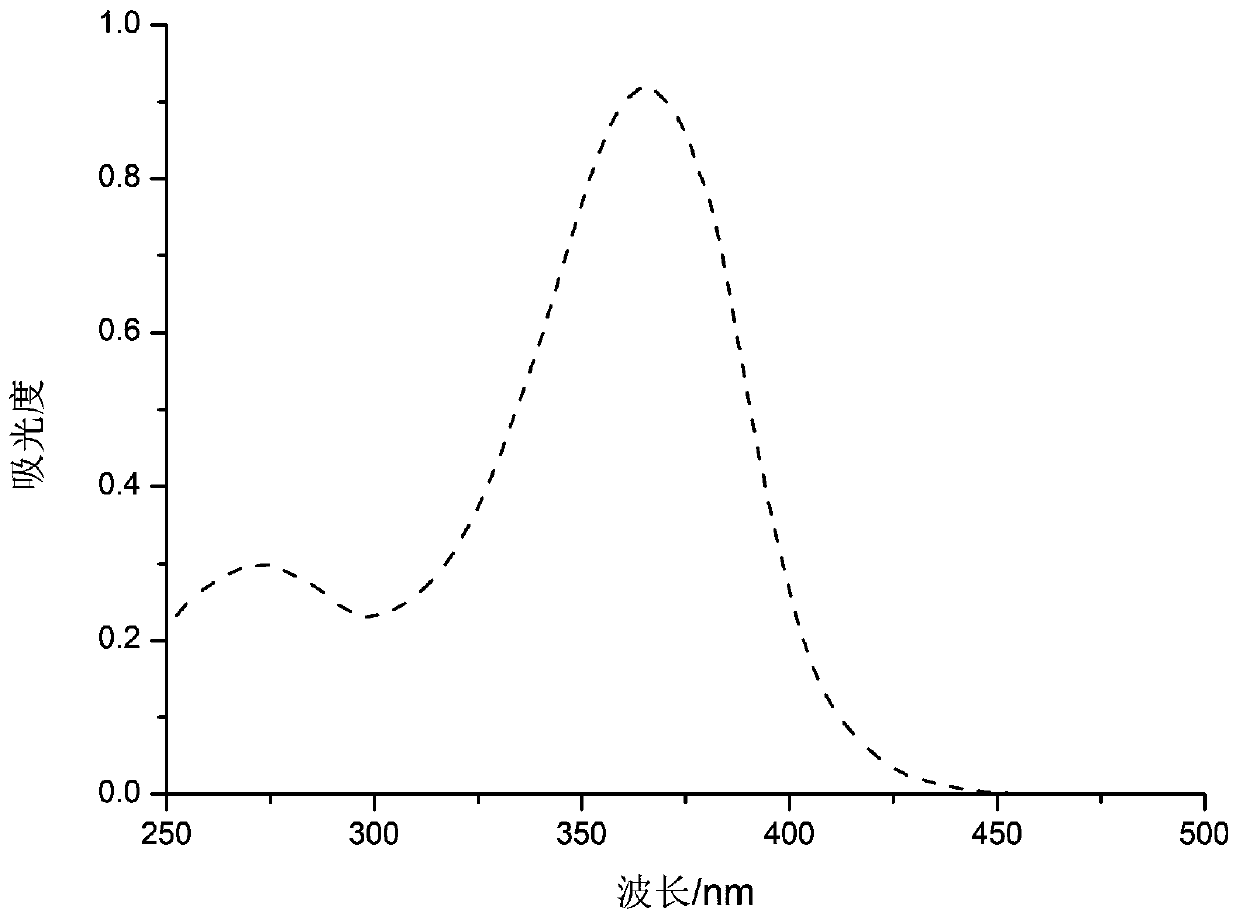
Long-wavelength methyl benzoylformate photoinitiator and preparation method thereof
PendingCN111559963AImprove performanceStrong market potentialOrganic compound preparationCarboxylic acid esters preparationOrganic synthesisKetone
The invention relates to the technical field of organic synthesis, and especially relates to a long-wavelength methyl benzoylformate photoinitiator and a preparation method thereof. The emission wavelength range of LED light sources used in the market at present is 365 nm or above. The maximum absorption wavelength of a traditional photoinitiator MBF is 255 nm, the wavelength is not matched with the emission wavelength of the LED light source, so in order to enable the maximum absorption wavelength of the MBF photoinitiator to be matched with the main emission wavelength range of the LED lightsource, the invention provides the long-wavelength methyl benzoylformate type photoinitiator and the preparation method thereof. The long-wavelength methyl benzoylformate photoinitiator is obtained by a condensation reaction of an aldehyde group and / or R1 functionalized methyl benzoylformate derivative and a ketone compound containing alpha H atoms, the maximum absorption wavelength of the long-wavelength methyl benzoylformate photoinitiator can reach 365 nm or above, and the long-wavelength methyl benzoylformate photoinitiator is matched with the LED light source used in the market and has good commercial application prospects.
Owner:HANGZHOU INST OF ADVANCED MATERIAL BEIJING UNIV OF CHEM TECH
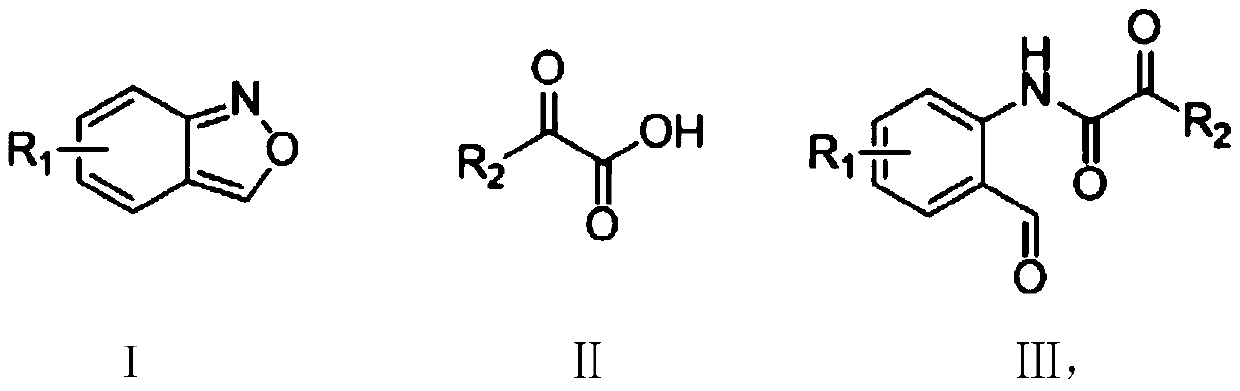
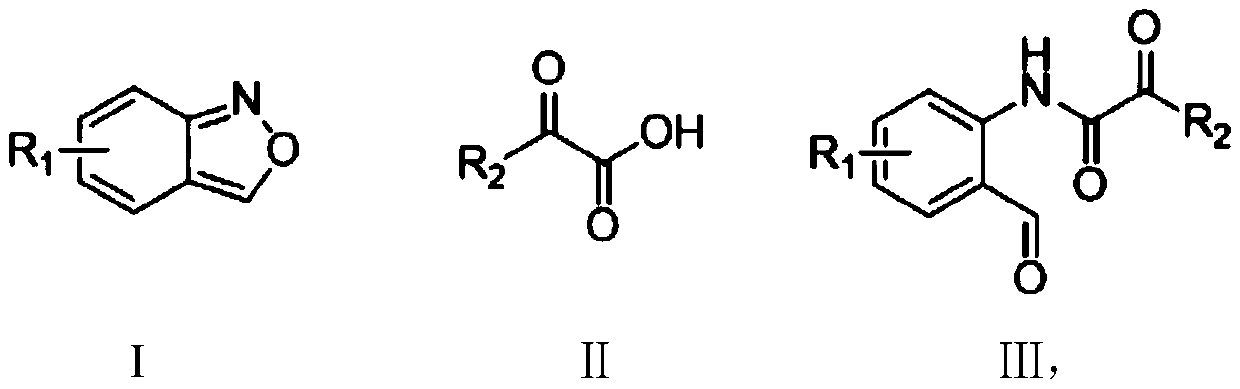
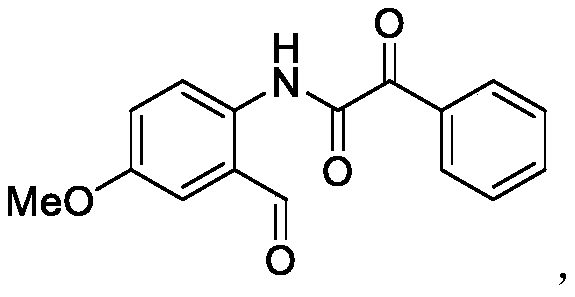
Method for synthesizing ortho-aldehyde group-containing alpha-ketoamide compound
ActiveCN109776488ALow priceReduce dosageOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsBenzeneOrganic solvent
The invention discloses a method for synthesizing an ortho-aldehyde group-containing alpha-ketoamide compound. Benzo[c]isoxazole shown in formula I and benzoyl formic acid shown in formula II are dissolved in an organic solvent and react in an inert atmosphere under the function of a catalyst, and an obtained reaction liquid is purified to obtain the ortho-aldehyde group-containing alpha-ketoamidecompound shown in formula III. The method has the advantages that the adopted raw materials are low in cost, the method is environmentally friendly, the use quantity of the catalyst is small, the catalytic efficiency is high, and the application range of substrates is wide. The formulas are show in the description.
Owner:JIANGNAN UNIV
Novel methyl benzoylformate initiator and preparation method thereof
InactiveCN104610541AImprove solubilityHigh activityOrganic compound preparationCarboxylic acid esters preparationArylHydrogen
The invention discloses a novel methyl benzoylformate initiator and a preparation method thereof. The general formula of the novel methyl benzoylformate initiator is as shown by a compound (1), a compound (2), a compound (3) and a compound (4) in the specification, wherein the substituent group R1 is selected from hydrogen, aryl, C1-C12 linear (branched) alkyls or alkoxys, C3-C12 naphthenic bases, and C4-C12 naphthenic alkyls, R2 is selected from aryl, C1-C12 linear (branched) alkyls, C3-C12 naphthenic bases, and C4-C12 naphthenic alkyls, and the substituent groups R3 and R4 are selected from C1-C12 linear (branched) alkyls. The invention provides a reactive and water-soluble methyl benzoylformate free radical initiator for taking part in photo-polymerization in an olefinic unsaturated compound system, and meanwhile, aims at thoroughly solving the problems of volatilization and migration.
Owner:TIANJIN MOSEN TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com