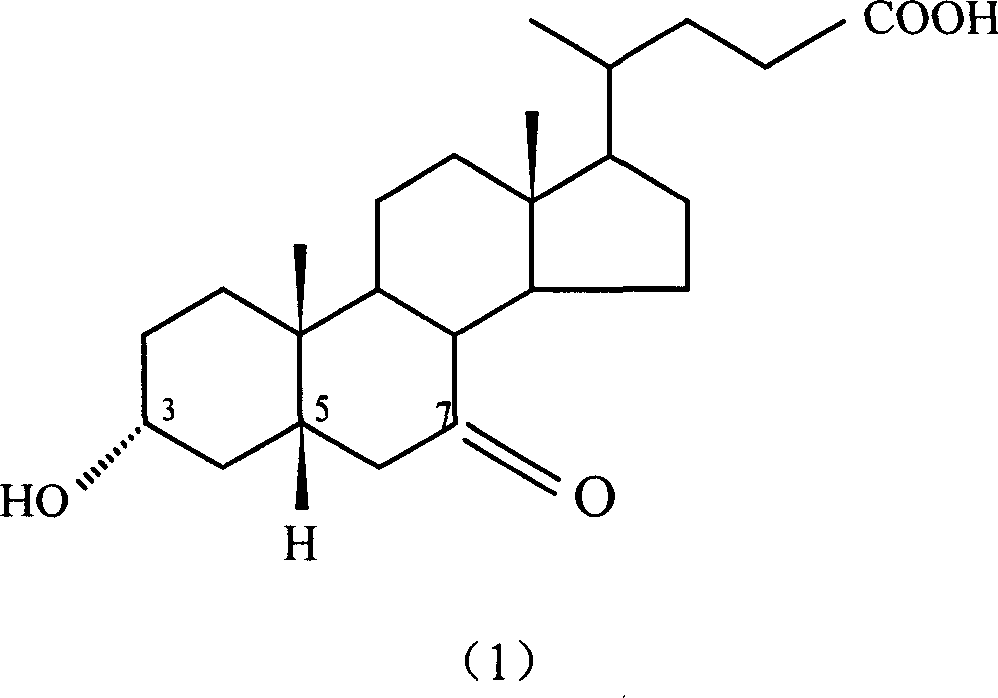
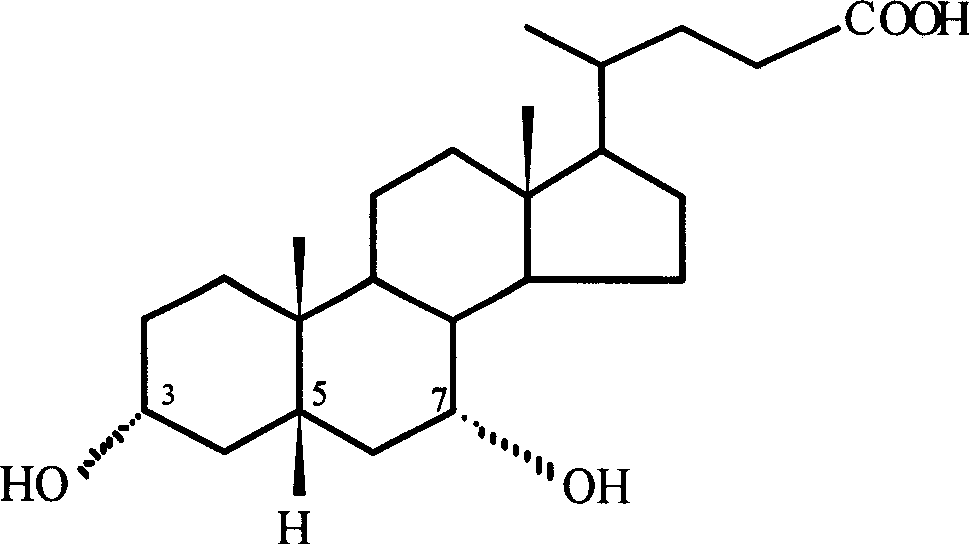
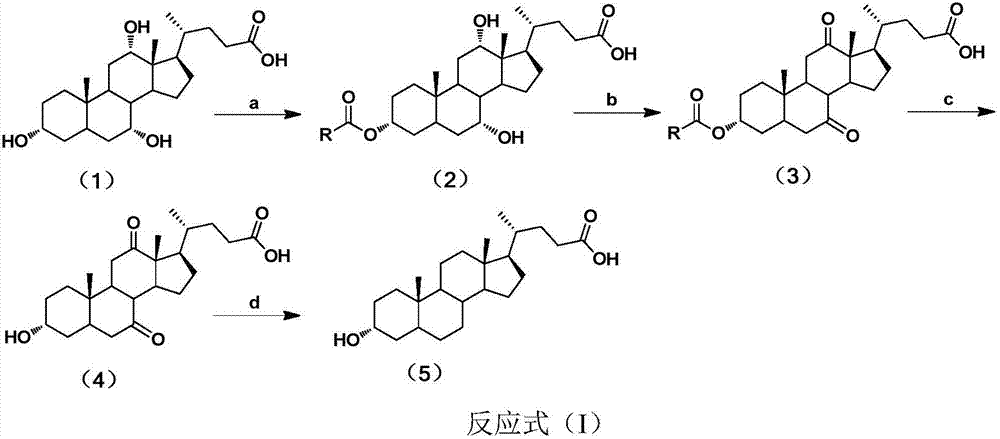
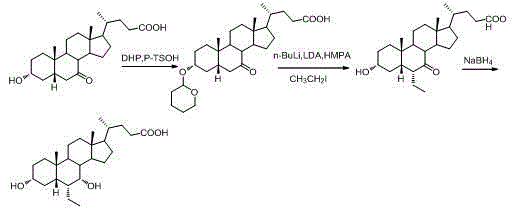
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
113 results about "Lithocholic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
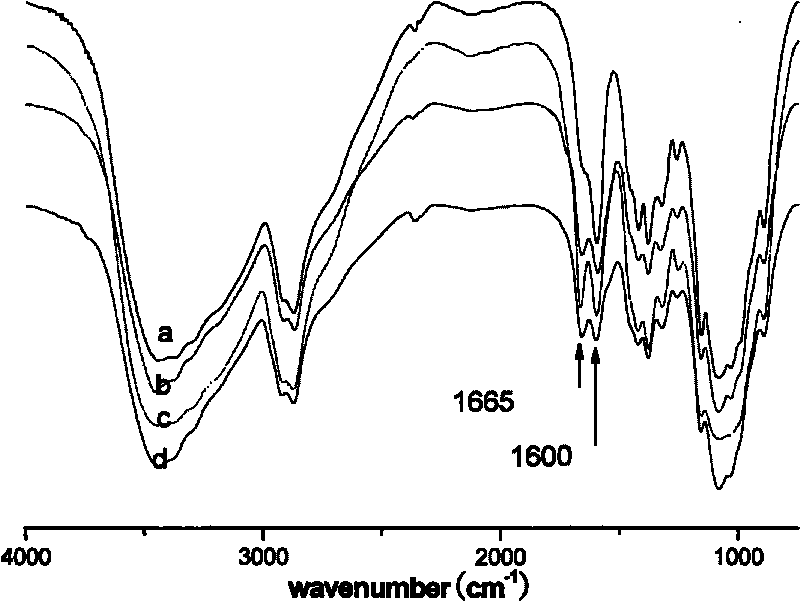
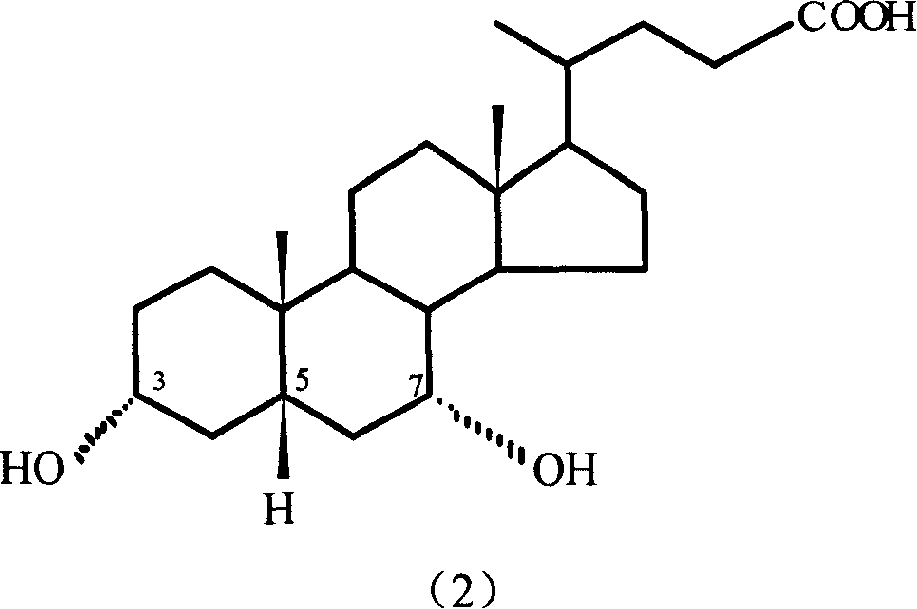
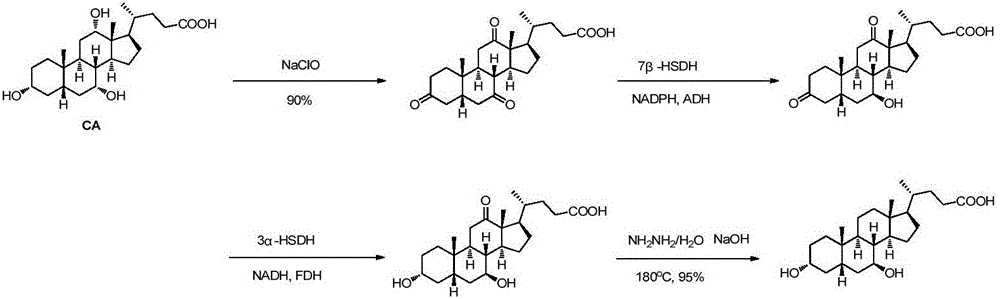
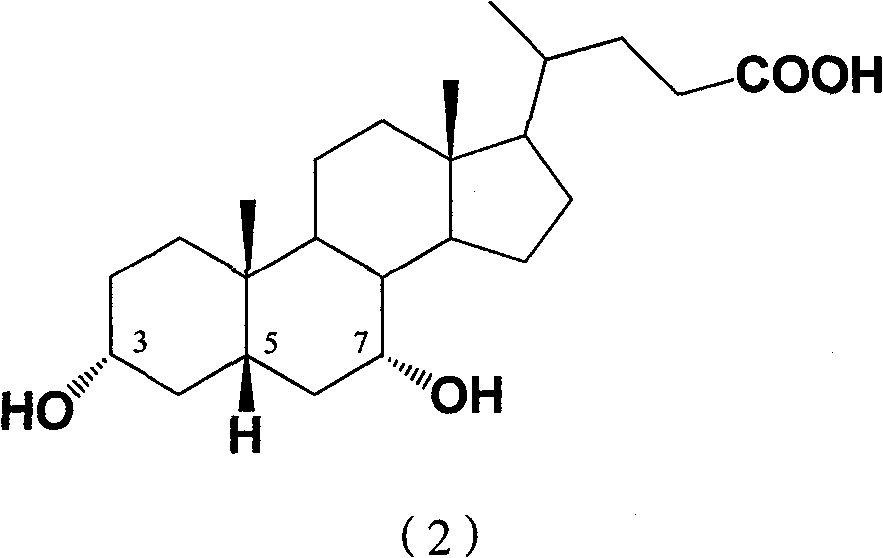
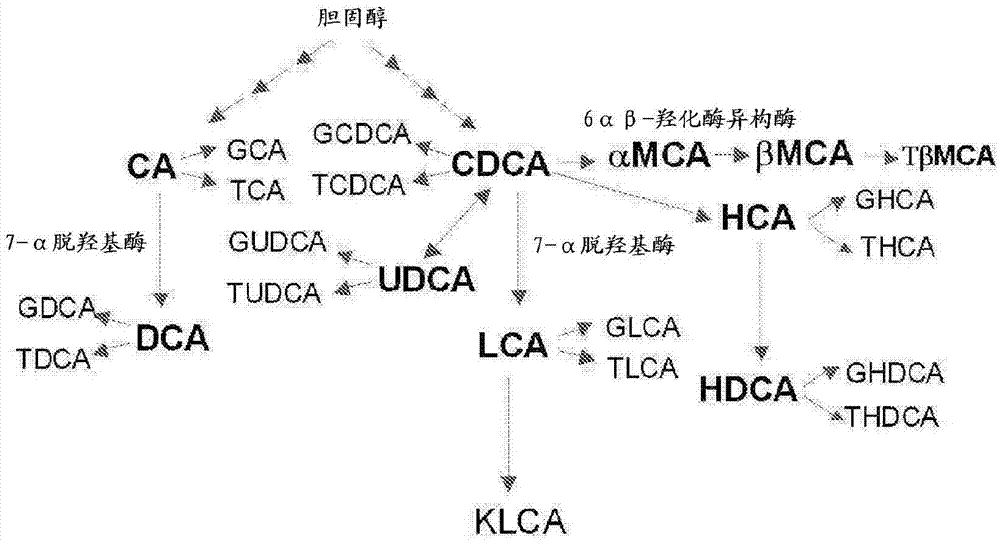
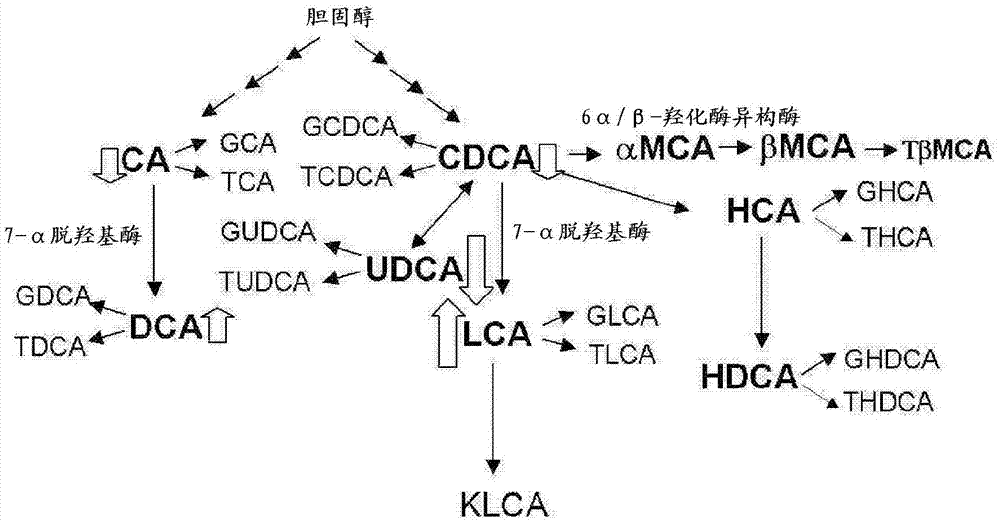
Lithocholic acid, also known as 3α-hydroxy-5β-cholan-24-oic acid or LCA, is a bile acid that acts as a detergent to solubilize fats for absorption. Bacterial action in the colon produces LCA from chenodeoxycholic acid by reduction of the hydroxyl functional group at carbon-7 in the "B" ring of the steroid framework.
Production method for extracting chenodeoxycholic acid using chicken gall
InactiveCN1850846AEasy to separateSimple processUnknown materialsSteroidsCholic acidChenodeoxycholic acid
The feature of the invention includes: adopting frozen chicken gallbladder slicing, heating to 70 degree centigrade, adding 10% weight of the bile boiling for 24 hours, reflux cooling, adjusting the pH value by hydrochloric acid to gain cream, washing to neutrality; adding 95% alcohol and 10% diatomite, reflux cooling and adding active carbon, reflux, cooling and filtering, adding petrol to the filtrate to take degreasing, reflux, standing, separating alcohol liquid and washing to neutrality in reaction kettle, adding 95% alcohol, after resolving, adding barium chloride and active carbon, reflux, hot filtering, concentrating white crystal from the filtrate, washing, and adding water and sodium carbonate water solution, heating and reflux, filtering, adjusting the pH value, drying. The invention has simple technology, low cost, and could drastically separate cholesterol, lithocholic acid, and cholic acid from gall, and improves the purity.
Owner:辽宁百隆生物工程有限公司
Method for preparing amphiphilic chitosan nanometer medicament carrier
ActiveCN101711873AEasy to operateControllable outputPharmaceutical non-active ingredientsLithocholic acidSolubility
The invention relates to an amphiphilic chitosan material prepared by grafting lipophilic micro-molecular lithocholic acid on chitosan molecules and having good biocompatibility and degradability. Under catalysis of catalysts EDC and NHS, Mv64-230KDa amphiphilic macromolecular chitosan and lipophilic micro-molecular lithocholic acid are subjected to amidation reaction to form the amphiphilic chitosan material with good biocompatibility and degradability; in acidic solution with pH of less than 6.5, the amphiphilic chitosan material can rapidly form uniformly-distributed nanometer micelle with granularity of between 200 and 400 nm based on a molecule self-assembling principle under the ultrasonic condition; and an internal amphiphilic structure of the amphiphilic chitosan material is favorable for improving solubility of fat-soluble medicaments and further the bioavailability of the medicament in human body is expected to be improved..
Owner:ZHANGJIAGANG IND TECH RES INST CO LTD DALIAN INST OF CHEM PHYSICS CHINESE ACADEMY OF SCI
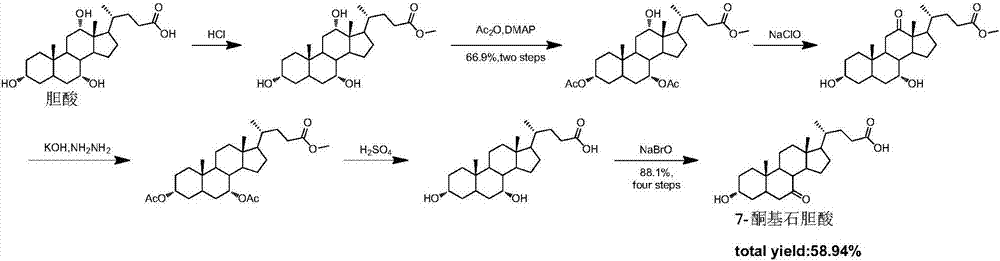
Preparation method of 7-keto lithocholic acid
InactiveCN1912192ASimple preparation stepsMild operating conditionsElectrolysis componentsElectrolytic organic productionLithocholic acidElectrolyte
The invention relates to 7-alkone lithocholic acid preparing method. It includes the following steps: dissolving the compound into the organic solvent to form electrolyte, electrolyzing the electrolyte by constant current at oxidizing medium condition to gain the object. The electrolyzing current density is 47.6A / m2-190.4A / m2. The invention has the advantages of moderate operation condition, green, simplifying ursodesoxycholic acid preparing steps.
Owner:EAST CHINA UNIV OF SCI & TECH
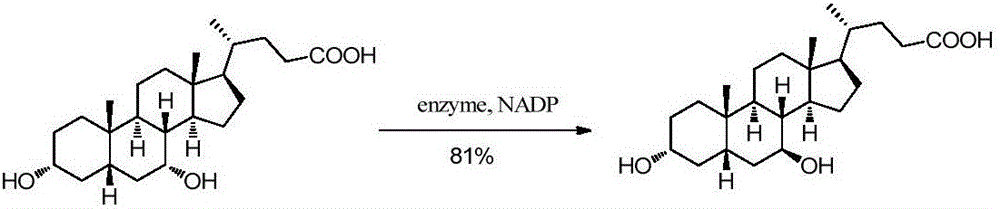
Chemical-enzymatic preparation of UDCA
The invention discloses a chemical-enzymatic preparation of UDCA. The chemical-enzymatic preparation of UDCA is characterized by comprising the following synthesis procedures: a) oxidizing UDCA under the action of 12alpha-HSDH to obtain 12-ketodeoxycholic acid, b) carrying out a decarbonylation reduction reaction on 12-ketodeoxycholic acid to obtain lithocholic acid, and c) carrying out hydroxylation on the lithocholic acid under the action of 7alpha-LCAH to obtain UDCA. Compared with the prior art, the chemical-enzymatic preparation of UDCA disclosed by the invention is low-cost and readily available in raw materials, mild in reaction conditions, less in steps and simple in post-treatment, and the substrate concentration reaches up to 100g / L, the total yield is 73%, the product purity is more than 99%, and the industrial large-scale production has very good application prospects.
Owner:SYNCOZYMES SHANGHAI
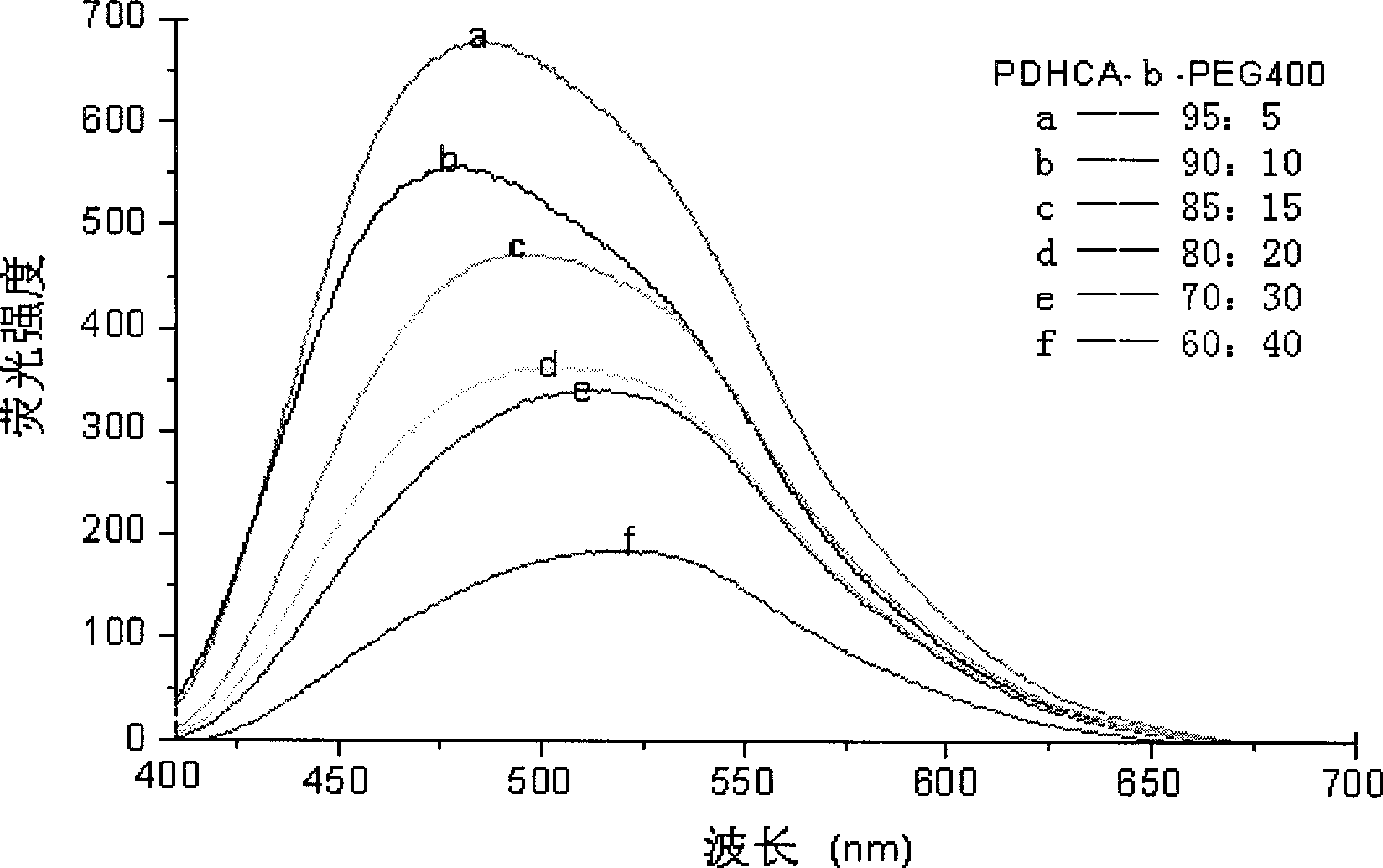
Preparation method for biodegradable fluorescent polyester multipolymer
InactiveCN101544749AMolecular weight controllableControllable glass transition temperatureSodium acetatePolyester
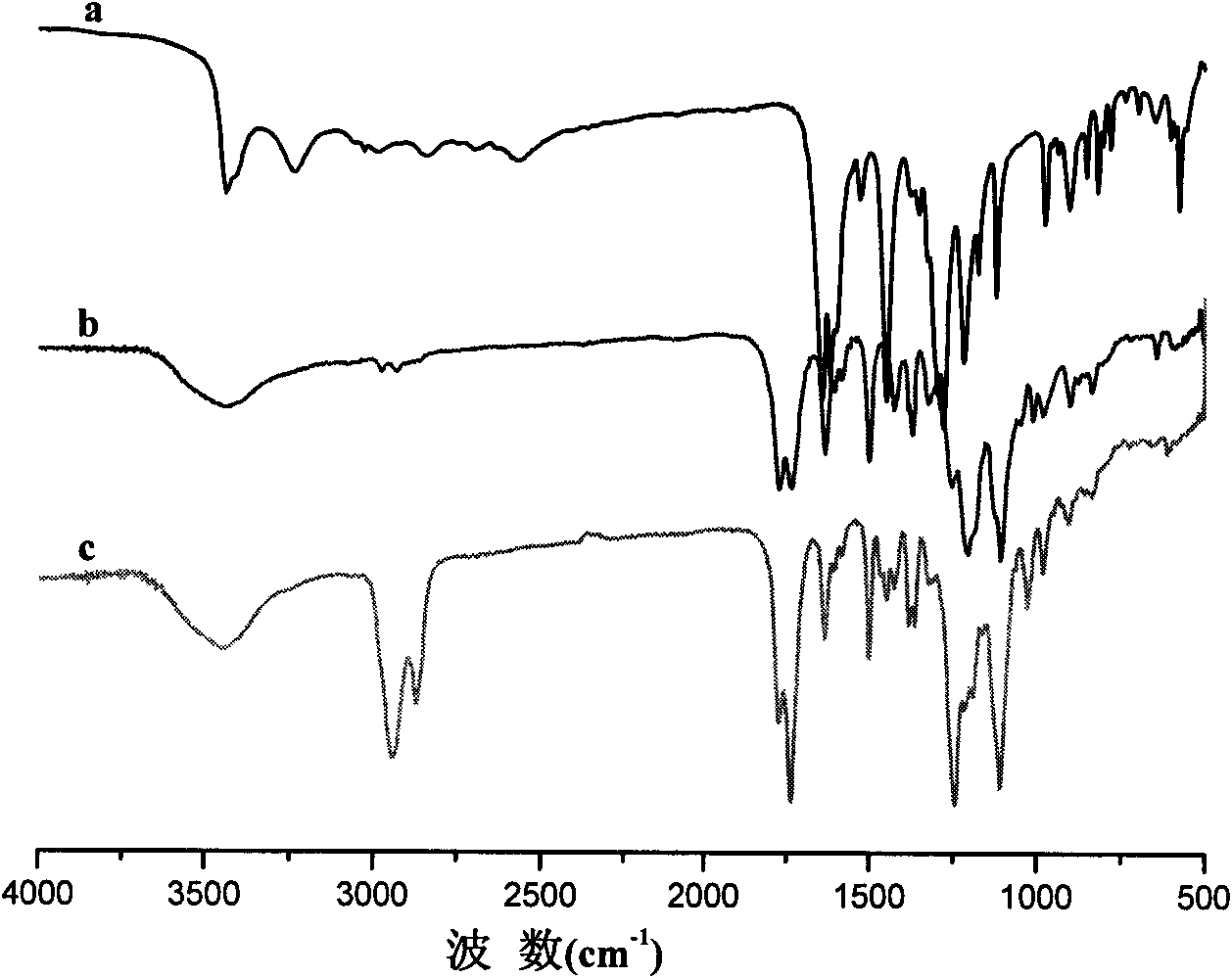
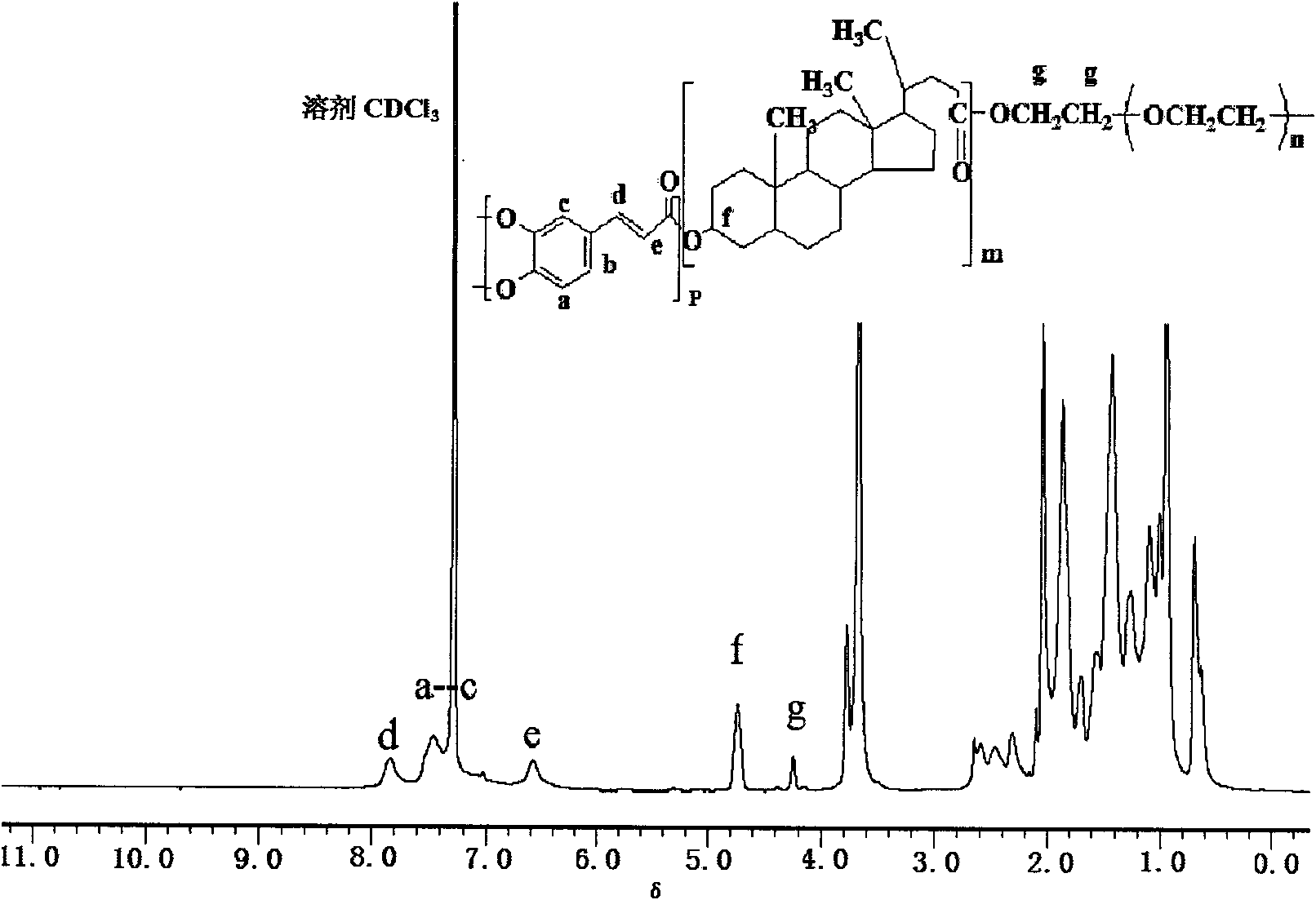
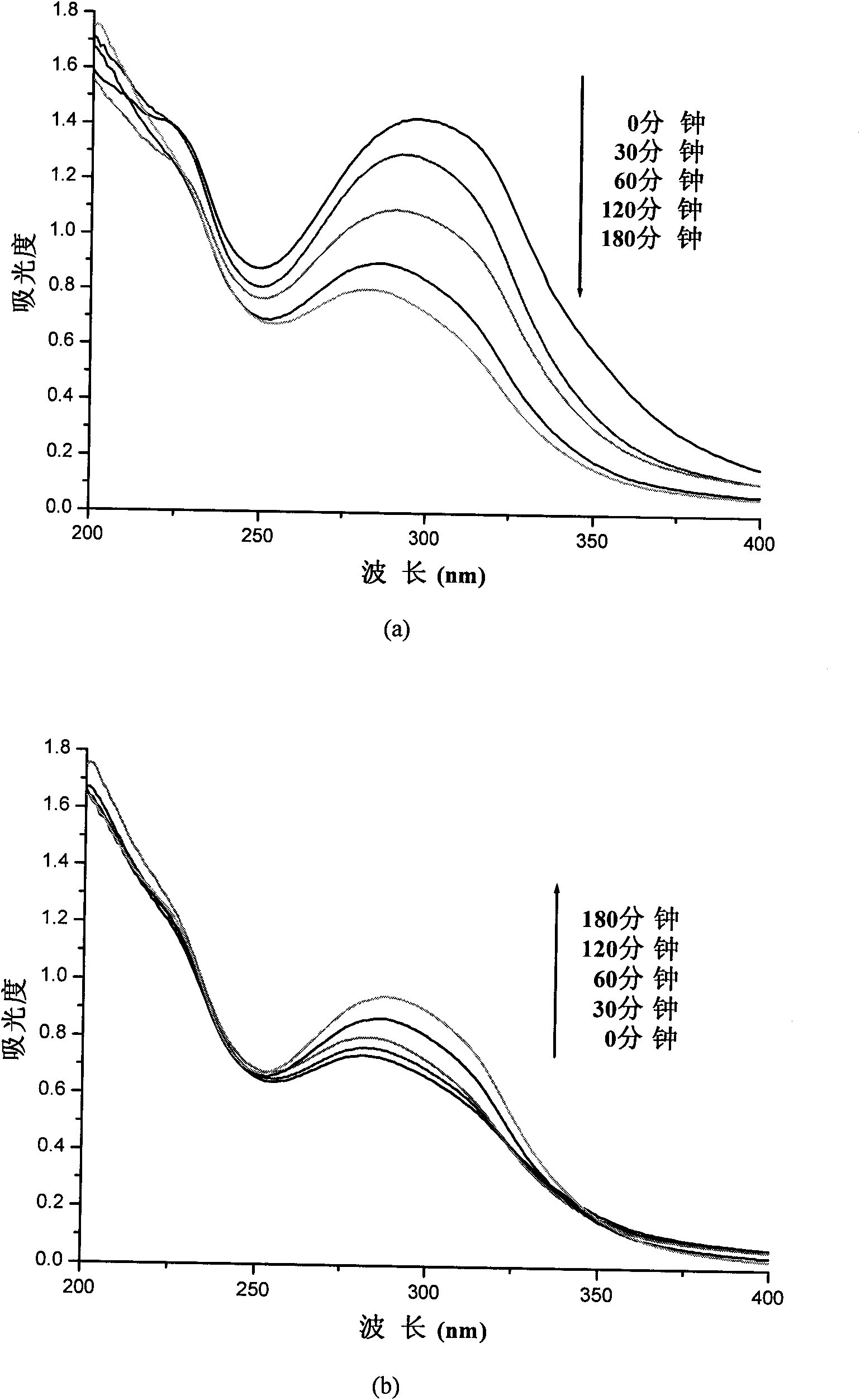
The invention relates to a preparation method for a biodegradable fluorescent polyester copolymer, belonging to the field of functional materials. The method comprises the following steps: using 3,4-dihydroxy cinnamic acid as a main monomer, using PEG400, PEG1000, PEG2000, PEG4000 and PEG6000 with different molecular weights, lactic acid, p-hydroxybenzoic acid or lithocholic acid as functional monomers and using sodium acetate as a catalyzer and acetic anhydride as a solvent; and adopting a two-step melting polycondensation method to obtain a caffeic acid ester copolymer with controllable molecular weight, glass transition temperature, fluorescence intensity, degradation speed, and the like so that the caffeic acid ester copolymer can be used as a degradation material or a fluorescent probe and applied to the fields of organizational projects, biological medicines, environmental protection, and the like. The invention provides a simple convenient high-efficiency method for preparing the biodegradable copolymer which has fluorescent performance.
Owner:JIANGNAN UNIV
Method for synthesizing lithocholic acid from hyodesoxycholic acid
ActiveCN106977572AReduction reaction with few side reactionsSimple post-processingSteroidsSynthesis methodsHyodeoxycholic acid
The invention discloses a method for synthesizing lithocholic acid, comprising: using hyodesoxycholic acid as a start material, and performing two-step reaction of 6Alpha-OH selective oxidation and Huang Minglon reduction to synthesize the lithocholic acid. The start material herein is low in price and easy to obtain, the synthetic steps are short, posttreatment is simple, few side reactions are employed, and the method is applicable to industrial production.
Owner:JIANGSU JIAERKE PHARMA GRP CORP
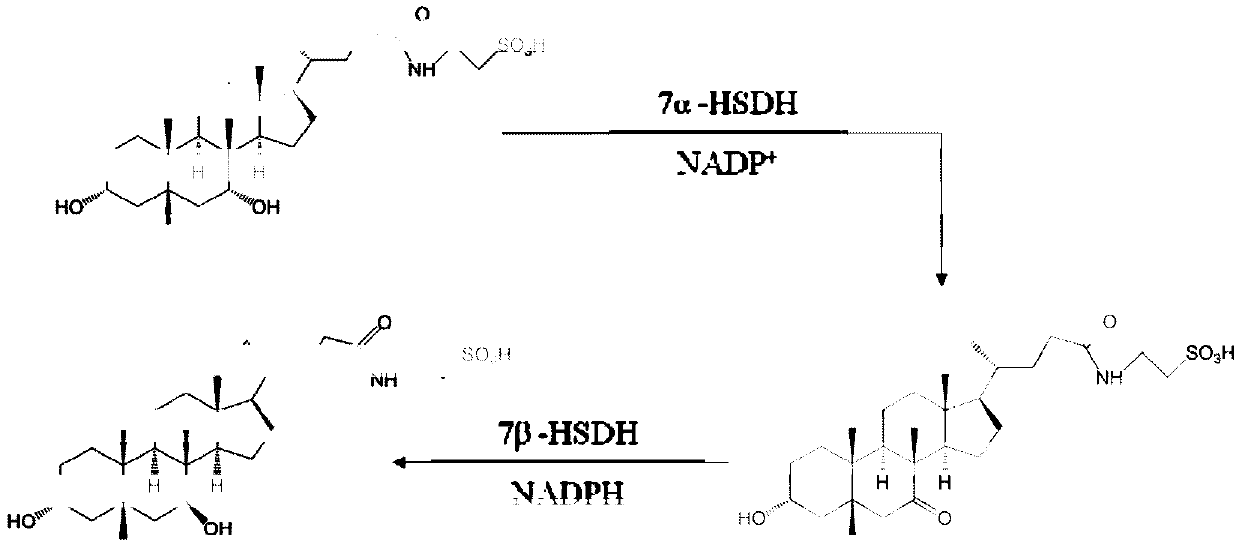
7beta-hydroxysterol dehydrogenase mutant and application of 7beta-hydroxysterol dehydrogenase mutant in ursodeoxycholic acid synthesis
ActiveCN107099516AEasy to separate and extractOvercoming the problem of inactivation processingOxidoreductasesGenetic engineeringChenodeoxycholic acidSubstrate concentration
The invention discloses a 7beta-hydroxysterol dehydrogenase mutant with increased activity and stability which is obtained through molecular evolution, recombinant expression plasmid containing the 7beta-hydroxysterol dehydrogenase mutant gene and a recombinant expression transformant and a preparation method of a recombinant mutant enzyme preparation, and the invention also provides an application of the recombinant mutant enzyme preparation in ursodeoxycholic acid synthesis. The 7beta-hydroxysterol dehydrogenase has excellent activity and heat stability, can efficiently catalyze asymmetric reduction of 7-carbonyl lithocholic acid to prepare the ursodeoxycholic acid; the 7beta-hydroxysterol dehydrogenase is subjected to immobilization and then is subjected to couple by an enzyme method with the immobilized 7beta-hydroxysterol dehydrogenase, epimerization of a substrate chenodeoxycholic acid with low cost can be directly catalyzed, ursodeoxycholic acid can be prepared through continuous conversion, and the operation is simple. Compared with the prior art reported currently, ursodeoxycholic acid prepared by hydroxysterol dehydrogenase through catalysis has the advantages of high substrate concentration, short reaction time, complete reaction, and high product purity, and has strong industrial application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Preparation method of 7-keto lithocholic acid
InactiveCN1912192BSimple preparation stepsMild operating conditionsElectrolysis componentsElectrolytic organic productionPower flowOrganic solvent
The invention relates to 7-alkone lithocholic acid preparing method. It includes the following steps: dissolving the compound into the organic solvent to form electrolyte, electrolyzing the electrolyte by constant current at oxidizing medium condition to gain the object. The electrolyzing current density is 47.6A / m<2>-190.4A / m<2>. The invention has the advantages of moderate operation condition, green, simplifying ursodesoxycholic acid preparing steps.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for synthesizing lithocholic acid from cholic acid
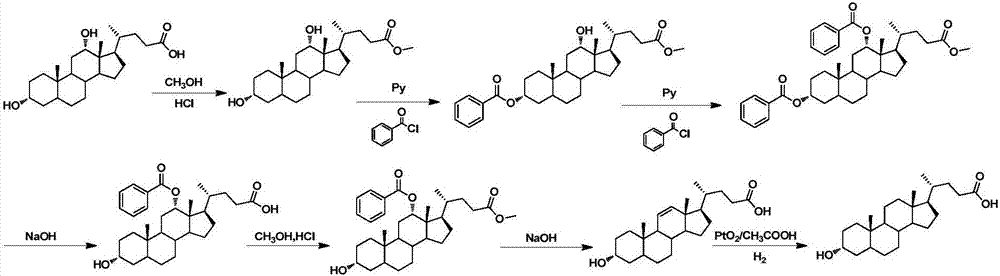
The invention discloses a method for synthesizing lithocholic acid from cholic acid. According to the method, cholic acid is used as a starting raw material, and lithocholic acid is synthesized through the following four steps of reaction: selective protection of 3alpha-OH; oxidation of 7alpha-OH and 12alpha-OH into carbonyl groups; hydrolysis; and Huang Min-lon reduction. The raw material used in the method is easily available and cheap, and the method is few in synthesis steps and side reactions, simple in after-treatment, high in overall yield and suitable for industrial production.
Owner:JIANGSU JIAERKE PHARMA GRP CORP
Method for preparing obeticholic acid intermediate
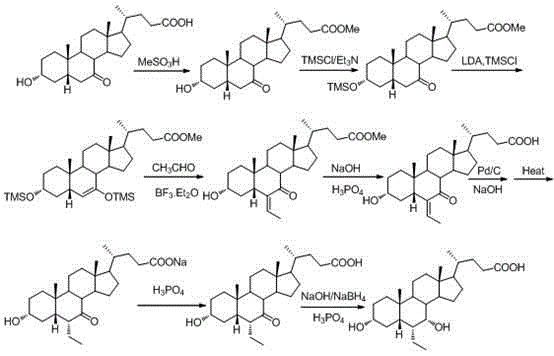
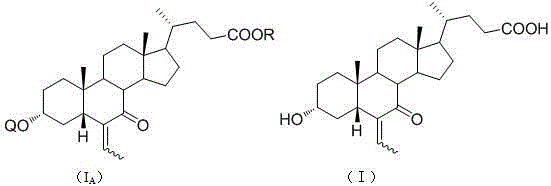
The invention discloses a method for preparing an obeticholic acid intermediate 3-alpha-hydroxyl-6-ethylidene-7-keto-5-beta-cholanic acid (I) by taking 7-oxo-lithocholic acid (II) as a raw material as well as its derivative (IA). According to the invention, 7-oxo-lithocholic acid (II) is protected or directly unprotected through 3-hydroxy or 3-hydroxy and 24-hydroxy, the is further subjected to an aldol condensation reaction with acetaldehyde to obtain the 3-alpha-hydroxyl-6-ethylidene-7-keto-5-beta-cholanic acid (I) and the derivative, and the method is used for preparing the obeticholic acid. The method has the advantages of simple process and high yield, and is suitable for industrial production.
Owner:CHONGQING PHARMA RES INST
Method for preparing ursodesoxycholic acid on basis of chemical oxidation and enzyme catalysis combination technology
ActiveCN106520888AReduce manufacturing costReduced activityFermentationEscherichia coliChenodeoxycholic acid
The invention discloses a new thought of generating 7-carbonyl-lithocholic acid by virtue of chenodeoxycholic acid dissolved by NBS acetone peroxide, and carrying out catalytic synthesis on the 7-carbonyl-lithocholic acid (7k-LCA) in combination with biological enzyme catalysis to obtain ursodesoxycholic acid (UDCA). The new thought is characterized by comprising the following steps: generating a catalytic enzyme (7 beta-HSDH) with high purity, high activity and high stability by virtue of the characteristics of high efficiency and low cost of the method of generating 7-carbonyl-lithocholic acid by virtue of chenodeoxycholic acid dissolved by NBS acetone peroxide and in combination with high-density fermentation of recombinant escherichia coli, and selectively catalyzing 7k-lithocholic acid in an aqueous phase to generate the ursodesoxycholic acid (UDCA). The synthesis process is simple, short in synthesis route, high in conversion rate and low in cost, and is a novel method for preparing ursodesoxycholic acid on the basis of a chemical oxidation and enzyme catalysis combination technology.
Owner:陕西岳达德馨生物制药有限公司
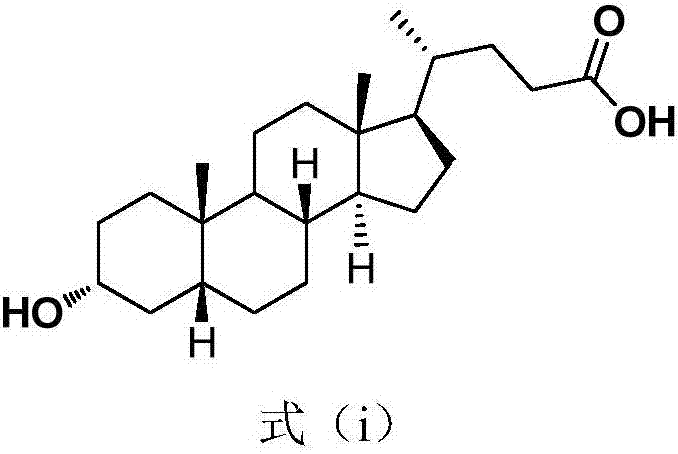
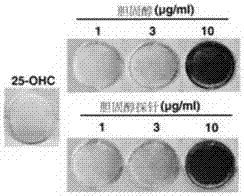
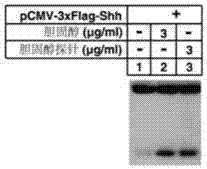
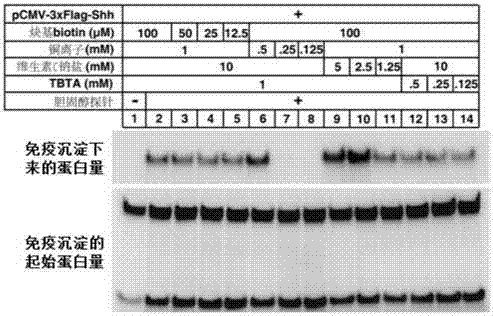
Cholesterol molecular probe as well as preparation method and application thereof
The invention discloses a cholesterol molecular probe and a preparation method thereof. The cholesterol molecular probe shown as a formula (I) is prepared by taking lithocholic acid as a raw material through esterification reaction, oxidization reaction, dehydrogenation reaction, carbonyl protection reaction, reduction reaction, hydroxyl protection reaction, reduction reaction, iodination reaction, substitution reaction and de-protection reaction. The invention further discloses application of the cholesterol molecular probe shown as the formula (I) to identification of cholesterol modified protein. The cholesterol molecular probe provided by the invention can be used for simulating normal cholesterol to promote cell growth, and prompting the shearing ripening of the cholesterol modified protein hedgehog, and can also be used for researching cholesterol modification of the protein.
Owner:WUHAN UNIV +1
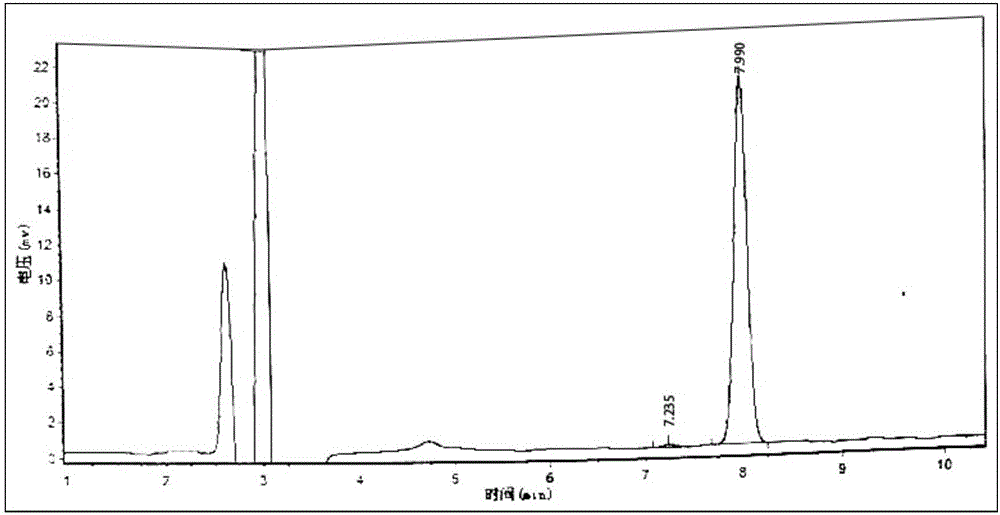
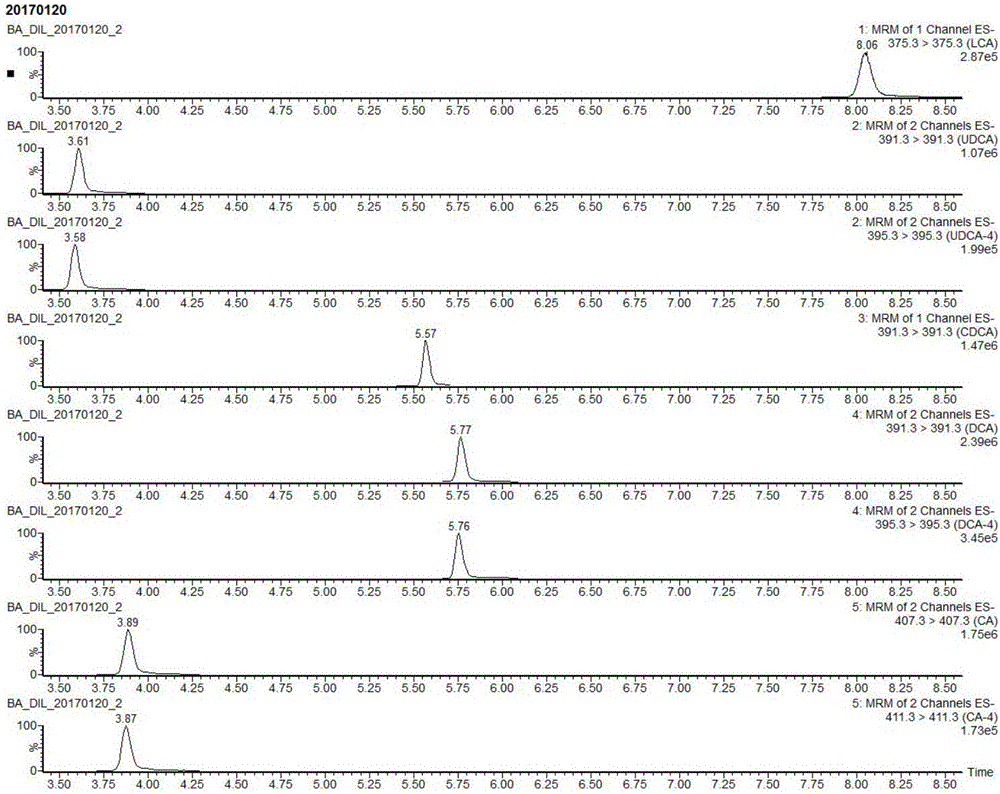
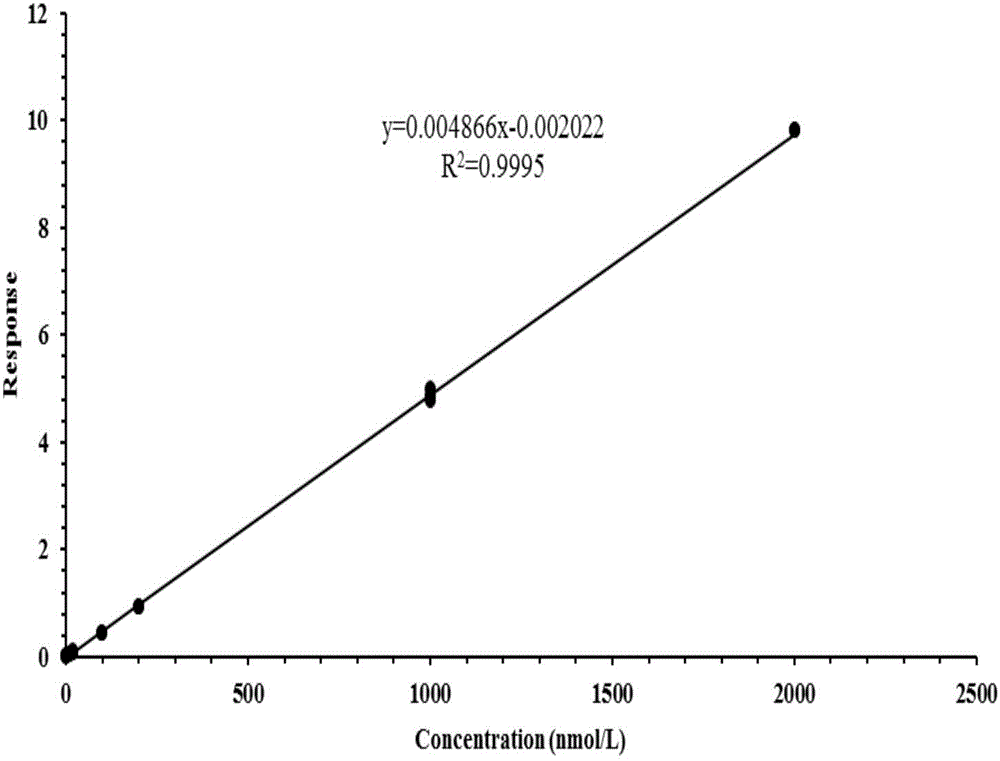
Method for detecting five free bile acids in serum through high performance liquid chromatography-tandem mass spectrum
InactiveCN106841492AEliminate distractionsSimplified processing stepsComponent separationCholic acidTandem mass spectrometry
The invention discloses a method for detecting five free bile acids in serum through high performance liquid chromatography-tandem mass spectrum. The five free bile acids comprises a cholic acid, a lithocholic acid, a deoxycholic acid, a ursodeoxycholic acid and a chenodesoxycholic acid; the method comprises the following steps of preparing a standard substance, performing high performance liquid chromatography separation, performing tandem mass spectrum detection, and detecting the free bile acids in the serum. The method can be used to simultaneously perform determination and accurately quantify the content of the free bile acids in human serum, the detection time is short, the flux is high, the detection sensitivity is high, and the specificity is good.
Owner:HANGZHOU BAICHEN MEDICAL LAB CO LTD
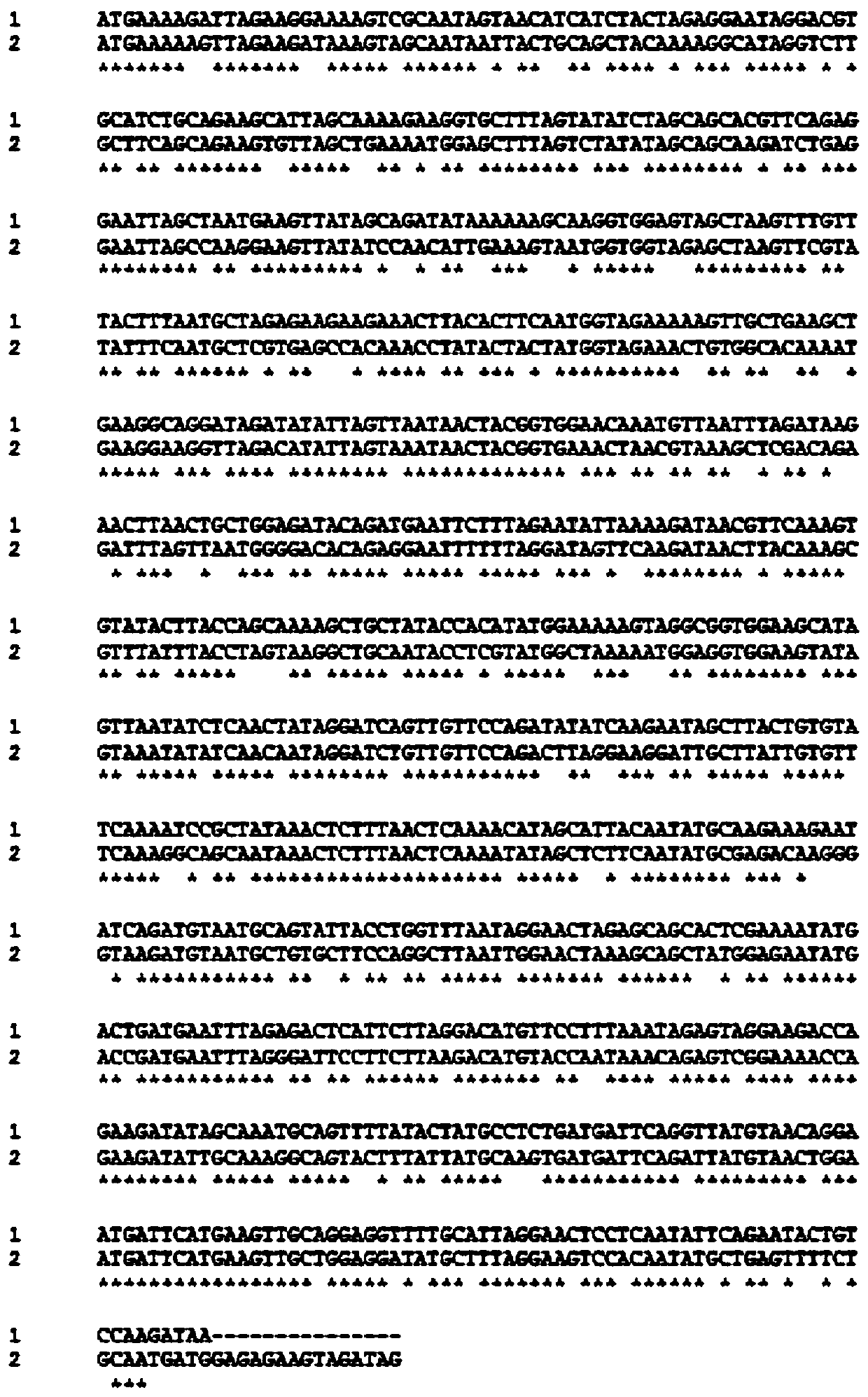
7 alpha-hydroxysteroid dehydrogenase as well as coding gene and application thereof
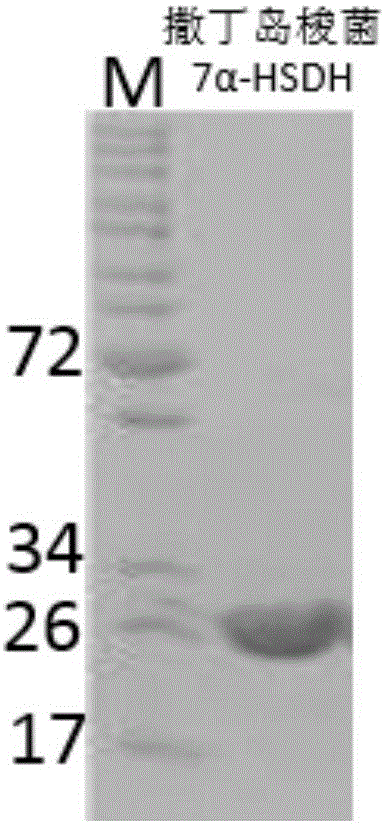
ActiveCN108034643ACatalytic asymmetric reduction reactionOxidoreductasesFermentationNucleotideKetone
The invention discloses 7 alpha-hydroxysteroid dehydrogenase as well as a coding gene and application thereof. An amino acid sequence of the 7 alpha-hydroxysteroid dehydrogenase provided by the invention is shown as SEQ ID NO. 1, and a nucleotide sequence of the 7 alpha-hydroxysteroid dehydrogenase gene is disclosed and shown as SEQ ID NO. 2. The 7 alpha-hydroxysteroid dehydrogenase can catalyze asubstrate taurocholic acid (TCA) to generate taurine 7-ketone cholic acid (T7K-CA), catalyze a substrate glycocholic acid (GCA) to generate glycine 7-ketone cholic acid (G7K-CA), catalyze a substratetaurochenodeoxycholic acid (TCDCA) to generate taurine 7-ketone lithocholic acid (T7K-LCA), catalyze a substrate glycochenodeoxycholic acid (GCDCA) to generate glycine 7-ketone lithocholic acid (G7K-LCA) and catalyze a substrate ethyl benzoylformate (EB) to generate ethyl 2-hydroxy-2-phenylacetate, and has better catalytic activity, has a catalytic activity to TCDCA, GCDCA and EB 10, 5, and 3 times that of sardinia clostridium 7 alpha-HSDH, correspondingly, and has great industrial application value.
Owner:CHONGQING UNIV
Method for preparing photoactive ternary amphiphilic polyester
The invention relates to a method for preparing photoactive ternary amphiphilic polyester, belonging to the functional material field. The invention uses lithocholic acid (LCA), cholic acid (CA), 3,4-dihydroxyl cinnamic acid (caffeic acid, DHCA), p-hydroxy-cinnamic acid (cumaric acid, 4HCA), lactic acid (LA), 1,3-propanediol (PDO) and polyethylene glycols (PEG) of different molecular weights as raw materials, anhydrous sodium acetate as a catalyst and acetic anhydride as a solvent, and adopts the stepwise polycondensation method to obtain the photoactive ternary amphiphilic polyester, the molecular weight, the glass transition temperature, the degradation speed and the photocrosslink degree of the obtained polyester are all controllable, and the amphiphilic polyester forms a micelle by self assembly. The preparation method is simple, and the prepared ternary amphiphilic polyester has good photoactivity, biological compatibility and thermal stability and can be widely used as degrading materials in the fields of medicinal slow-release systems, tissue engineering, packaging material, environmental protection and the like.
Owner:JIANGNAN UNIV +1
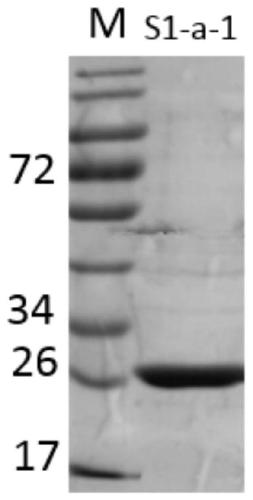
Gene S1-a-1 of novel 7 alpha-HSDH (hydroxysteroid dehydrogenase)
ActiveCN106701707ACatalytic carbonyl asymmetric reduction reactionBacteriaOxidoreductasesChenodeoxycholic acidNucleotide
The invention relates to HSDH (hydroxysteroid dehydrogenase), in particular to a gene S1-a-1 of novel 7 alpha-HSDH. A nucleotide sequence of the gene is shown as SEQ ID NO.2, the novel 7 alpha-HSDH is encoded, a nucleotide sequence of the novel 7 alpha-HSDH is shown as SEQ ID NO.1, the novel 7 alpha-HSDH can be used for catalyzing CDCA (chenodeoxycholic acid) and TCDCA (taurochenodeoxycholic acid) to generate 7K-LCA (7-ketone lithocholic acid) and T7K-LCA (taurine-7-ketone lithocholic acid), wherein the catalytic activity of the novel 7 alpha-HSDH for CDCA is about 5 times of that of 7 alpha-HSDH of Sardinia clostridium, and the catalytic activity of the novel 7 alpha-HSDH for TCDCA is about over 2.5 times of that of 7 alpha-HSDH of Sardinia clostridium, therefore, the novel 7 alpha-HSDH has a great industrial application value.
Owner:CHONGQING KINBEAR BIOTECHNOLOGY CO LTD
Medicine for promoting postoperative rehabilitation of facial nerve injury surgical anastomosis
InactiveCN103040976AReduce usageIncreased risk of postoperative bleedingNervous disorderInorganic active ingredientsClinical trialSurgical anastomosis
The invention discloses a medicine for promoting postoperative rehabilitation of facial nerve injury surgical anastomosis, which solves the postoperative rehabilitation problem of facial nerve injury surgical anastomosis. The medicine is characterized by being processed by a plurality of traditional Chinese medicines and comprises following raw materials by weight ratio: 10 to 20 parts of sunglo, 8 to 15 parts of filipendula palmate, 8 to 15 parts of herb of glabrous sarcandra, 6-10 parts of cineraria repanda, 6 to 10 parts of pawpaw, 8 to 15 parts of pangolin, 6 to 10 parts of cimicifugae foetidae, 3 to 6 parts of cassia twig and 3 to 6 parts of lithocholic acid. Clinical trials prove that the medicine for promoting postoperative rehabilitation of facial nerve injury surgical anastomosis has the characteristics of good curative effect and higher safety, and is worth to be clinically applied and popularized.
Owner:刘建贞
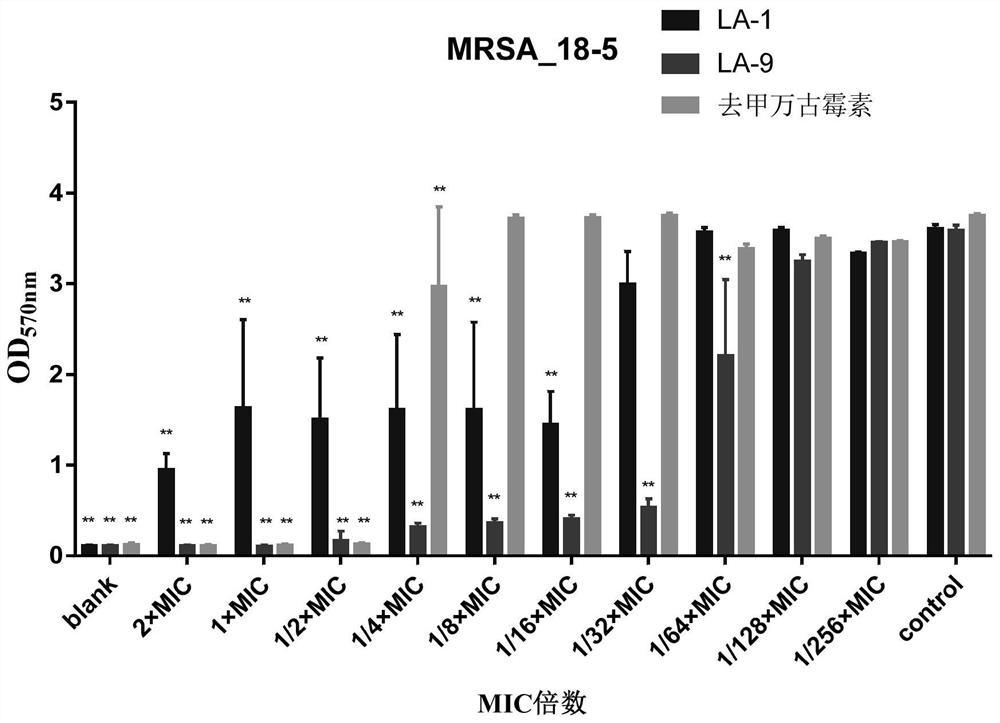
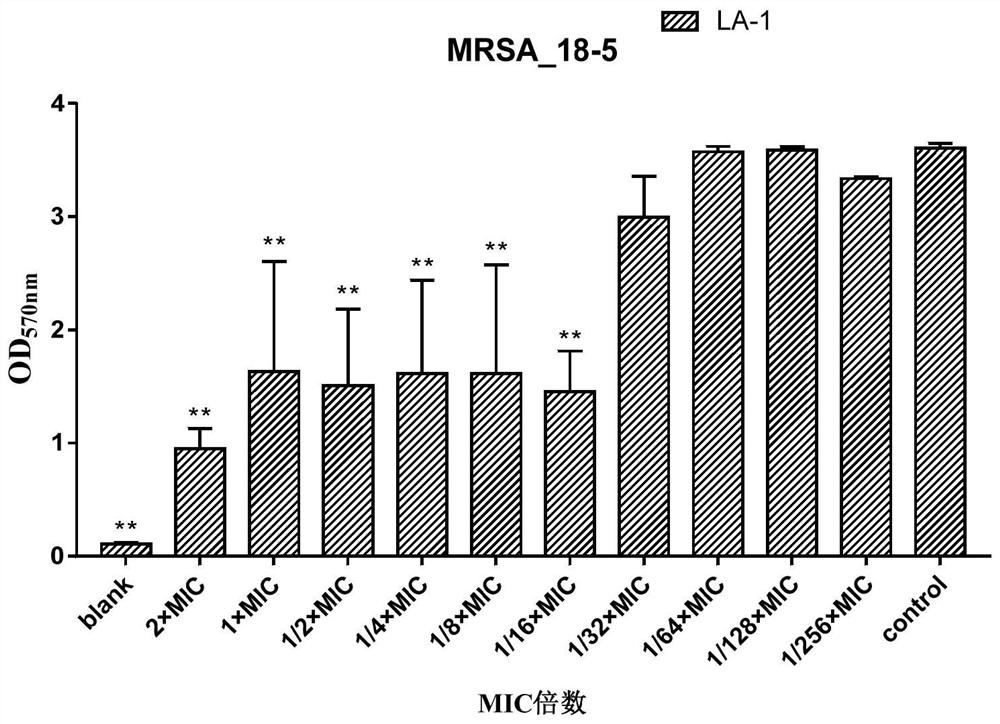
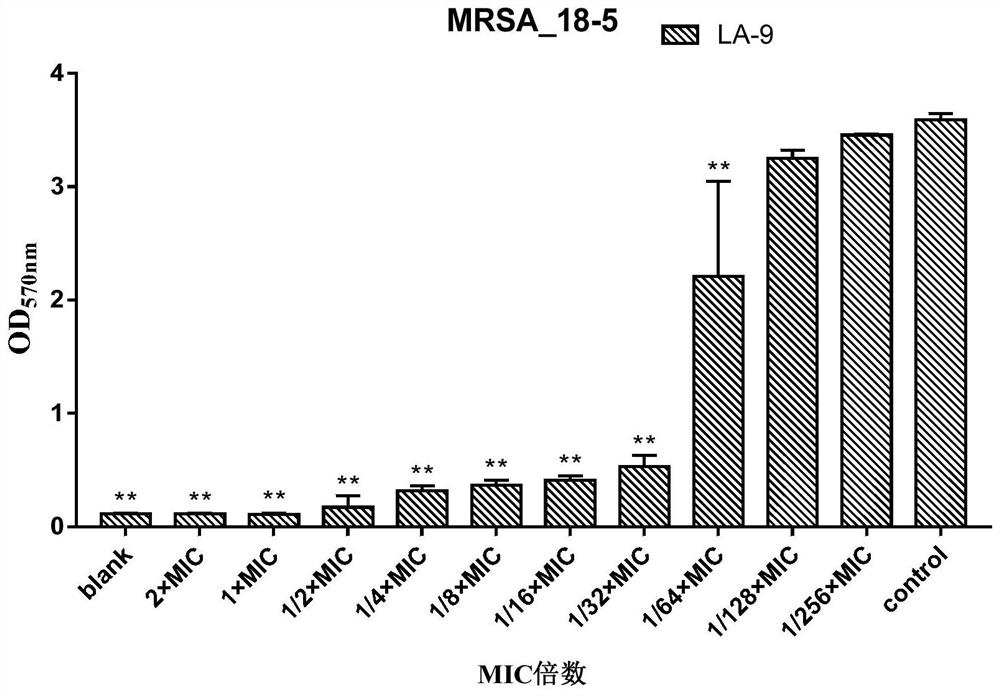
Application of lithocholic acid compound in preparation of antibacterial product
ActiveCN113559013AHigh antibacterial activityGood antibacterial effectAntibacterial agentsCosmetic preparationsBiotechnologyCholic acid
The invention discloses application of a lithocholic acid compound in preparation of an antibacterial product, belongs to the technical field of biological pharmacy, and discovers the application of a series of lithocholic acid compounds in the preparation of the antibacterial product for the first time. Experiments show that a compound LA-1, a compound LA-5 and a compound LA-9 have a good bacteriostatic activity, the bacteriostatic effects of the three compounds on MRSA and MSSA are superior to those of other tested bacteria, and the bacteriostatic activity of the compound LA-9 on MRSA and MSSA is better than that of the compound LA-1 and the compound LA-5. Meanwhile, further experiments show that the two compounds LA-1 and LA-9 perform the bacteriostatic activity on MRSA and MSSA through the effect of resisting the growth of a staphylococcus aureus biofilm.
Owner:南充市中心医院 +1
Method for removing lithocholic acid from chenodeoxycholic acid
The invention discloses a method for removing lithocholic acid from chenodeoxycholic acid. The method comprises steps as follows: dissolving, extraction separation, crude product drying and mother solution treatment of chenodeoxycholic acid, crystallization, drying of a finished product, leftover recycling and the like. According to the great dissolution difference of lithocholic acid, lipid-like substances and chenodeoxycholic acid in n-heptane, more than 90% of lithocholic acid and the lipid-like substances are extracted once, the operation is simple and efficient, moreover, chenodeoxycholic acid is non-loss, n-heptane has a higher boiling point and is not prone to volatilization, a butyl acetate and methylene dichloride mixed solvent is adopted for crystallization, the crystallization ratio of chenodeoxycholic acid, the dissolution rate of lithocholic acid and other hetero-cholic acid and the extraction rate of chenodeoxycholic acid are increased, biological non-renewable resources are utilized to the greatest extent, and better economic benefits and resource utilization rate are created.
Owner:CHANGDE YUNGANG BIOTECHNOLOGY CO LTD
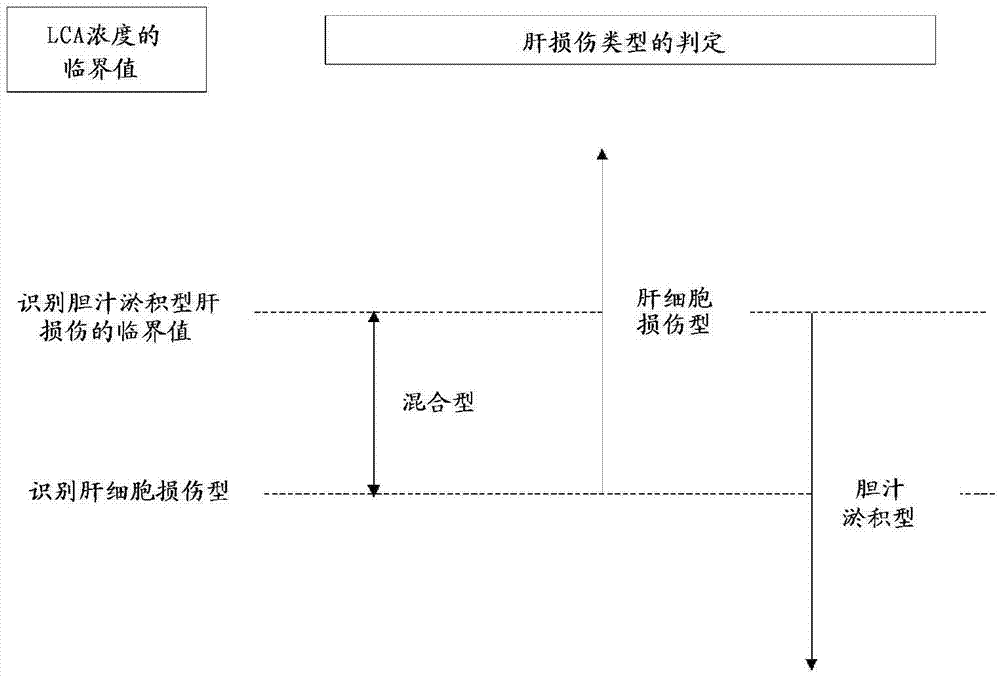
Method for investigation of liver damage type
Provided is an investigation method for determining liver damage type, using as an indicator the concentration of lithocholic acid (hereinafter abbreviated as LCA) in a biological sample derived from a test subject. Specifically, there is provided an investigation method in which the concentration of LCA in a biological sample taken from a test subject is measured, and using the measured LCA concentration as an indicator, the type of liver damage is determined to be hematocyte damage type, cholestatic type, or a mixed type thereof.
Owner:DAIICHI SANKYO CO LTD
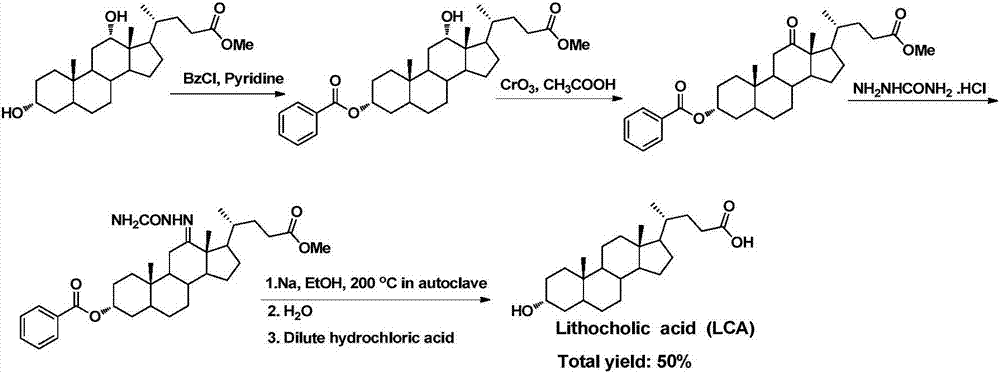
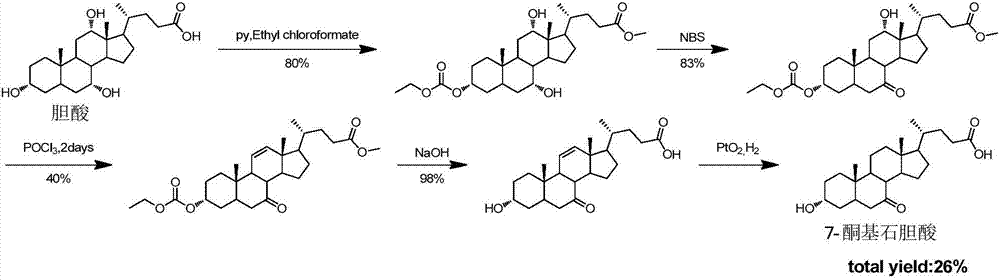
Synthetic method of 7-keto-lithocholic acid
The invention discloses a chemical synthetic method of an intermediate 7-keto-lithocholic acid (3alpha-hydroxyl-7-ketone-5beta-cholestane-24-acid) of obeticholic acid, and belongs to the field of organic chemical synthesis. According to the chemical synthetic method of the intermediate 7-keto-lithocholic acid (3alpha-hydroxyl-7-ketone-5beta-cholestane-24-acid) of obeticholic acid, cholic acid is adopted as a raw material, and through reactions of selective oxidization of 7alpha-hydroxyl, esterification of side chain carboxyl groups, esterification of 3alpha-hydroxyl, methanesulfonic acid esterification, elimination, hydrogenation, and hydrolysis of 12alpha-hydroxyl, the intermediate 7-keto-lithocholic acid (3alpha-hydroxyl-7-ketone-5beta-cholestane-24-acid) of obeticholic acid is synthesized. According to the chemical synthetic method of the intermediate 7-keto-lithocholic acid (3alpha-hydroxyl-7-ketone-5beta-cholestane-24-acid) of obeticholic acid, cheap cholic acid is adopted as the raw material, the synthesis method is novel, low in cost, high in yield and environmentally friendly, which facilitates industrialized production.
Owner:EAST CHINA NORMAL UNIV
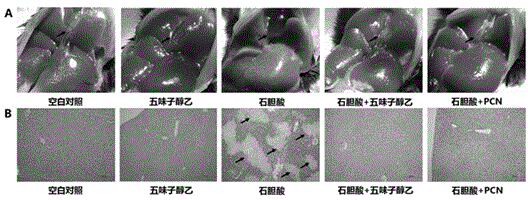
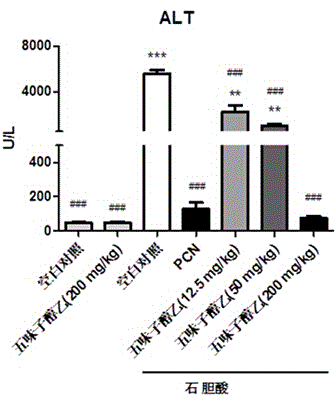
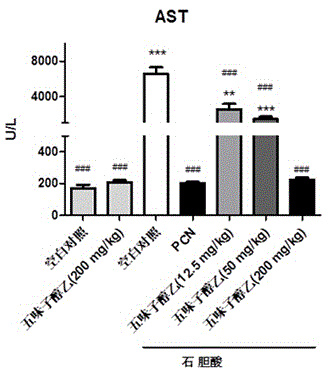
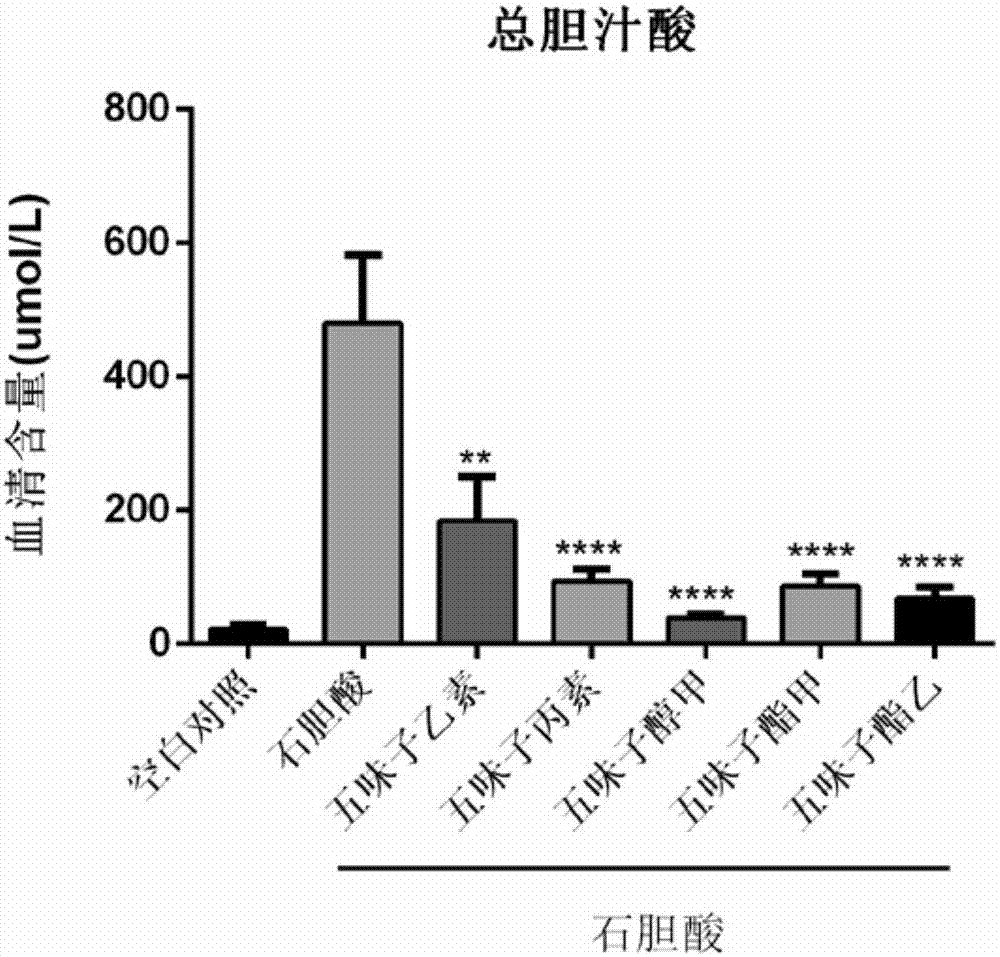
Application of schisandrol B in preparation of medicine for preventing and treating cholestatic liver disease
InactiveCN104083359AReduce necrosisReduced activityMetabolism disorderDigestive systemCholic acidBile Juice
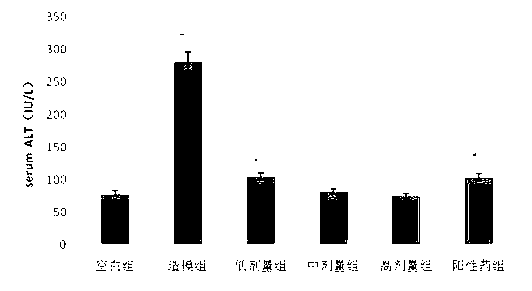
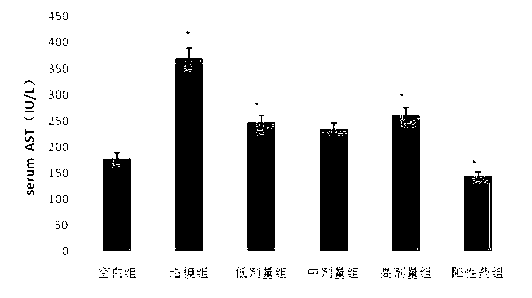
The invention discloses application of schisandrol B in preparation of a medicine for preventing and treating cholestatic liver disease. By giving an excess amount of lithocholic acid, a mice intrahepatic cholestasis model is made, or a mice extrahepatic cholestasis model is made by bile duct ligation, then the mice intrahepatic cholestasis model and the mice extrahepatic cholestasis model are respectively collaboratively given the schisandrol, liver injury can be effectively alleviated, the bile acid level is reduced, glutamic-pyruvic transaminase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) activity are decreased significantly, liver tissue necrosis degree is significantly alleviated, at the same time, total bile acid and total bilirubin content in serum are significantly reduced, and the total bile acid and total bilirubin content discharged with urine are increased. The results show that the schisandrol B can prevent and treat cholestatic liver injury induced by lithocholic acid and bile duct ligation, and prove that the effect mechanism may be involved in regulation of CYP3A11, UGT1A1 and OATP1A4 mediated bile acid metabolism and excretion.
Owner:SUN YAT SEN UNIV
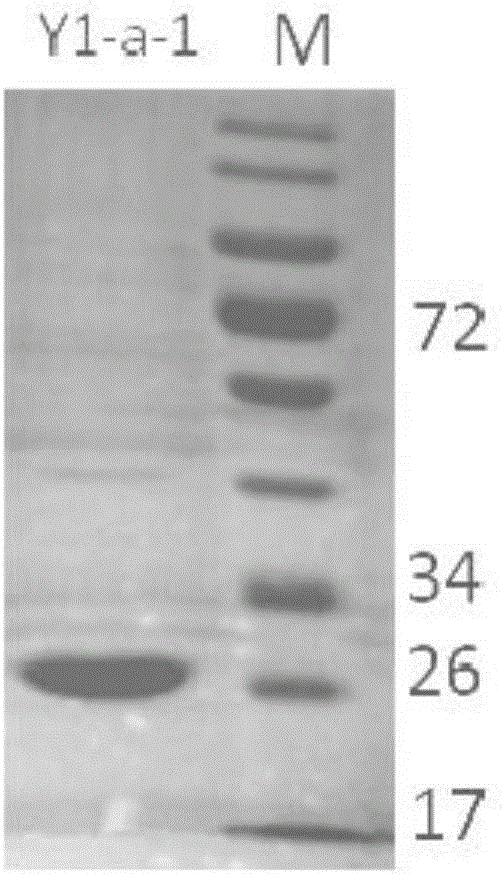
New 7alpha-hydroxysteroid dehydrogenase gene Y1-a-1
ActiveCN106701708AHigh industrial application valueBetter than activityBacteriaOxidoreductasesChenodeoxycholic acidNucleotide
The invention relates to hydroxysteroid dehydrogenase, in particular to a new 7alpha-hydroxysteroid dehydrogenase gene Y1-a-1. The nucleotide sequence of the gene is as shown in SEQ ID NO. 2. New 7alpha-hydroxysteroid dehydrogenase is coded, and the amino acid sequence of new 7alpha-hydroxysteroid dehydrogenase is as shown in SEQ ID NO.1; new 7alpha-hydroxysteroid dehydrogenase can catalyze chenodeoxycholic acid (CDCA) and taurochenodeoxycholic acid (TCDCA) to generate 7-ketone lithocholic acid (7K-LCA) and tauro 7-ketone lithocholic acid (T7K-LCA); the catalysis activity for CDCA or TCDCA is more than two times that of existing Sardinia clostridium 7alpha-HSDH, and the new 7alpha-hydroxysteroid dehydrogenase gene Y1-a-1 has an extremely large industrial application value.
Owner:CHONGQING UNIV
Production method for extracting chenodeoxycholic acid using chicken gall
InactiveCN1850846BEasy to separateSimple processUnknown materialsSteroidsCholic acidChenodeoxycholic acid
The feature of the invention includes: adopting frozen chicken gallbladder slicing, heating to 70 degree centigrade, adding 10% weight of the bile boiling for 24 hours, reflux cooling, adjusting the pH value by hydrochloric acid to gain cream, washing to neutrality; adding 95% alcohol and 10% diatomite, reflux cooling and adding active carbon, reflux, cooling and filtering, adding petrol to the filtrate to take degreasing, reflux, standing, separating alcohol liquid and washing to neutrality in reaction kettle, adding 95% alcohol, after resolving, adding barium chloride and active carbon, reflux, hot filtering, concentrating white crystal from the filtrate, washing, and adding water and sodium carbonate water solution, heating and reflux, filtering, adjusting the pH value, drying. The invention has simple technology, low cost, and could drastically separate cholesterol, lithocholic acid, and cholic acid from gall, and improves the purity.
Owner:辽宁百隆生物工程有限公司
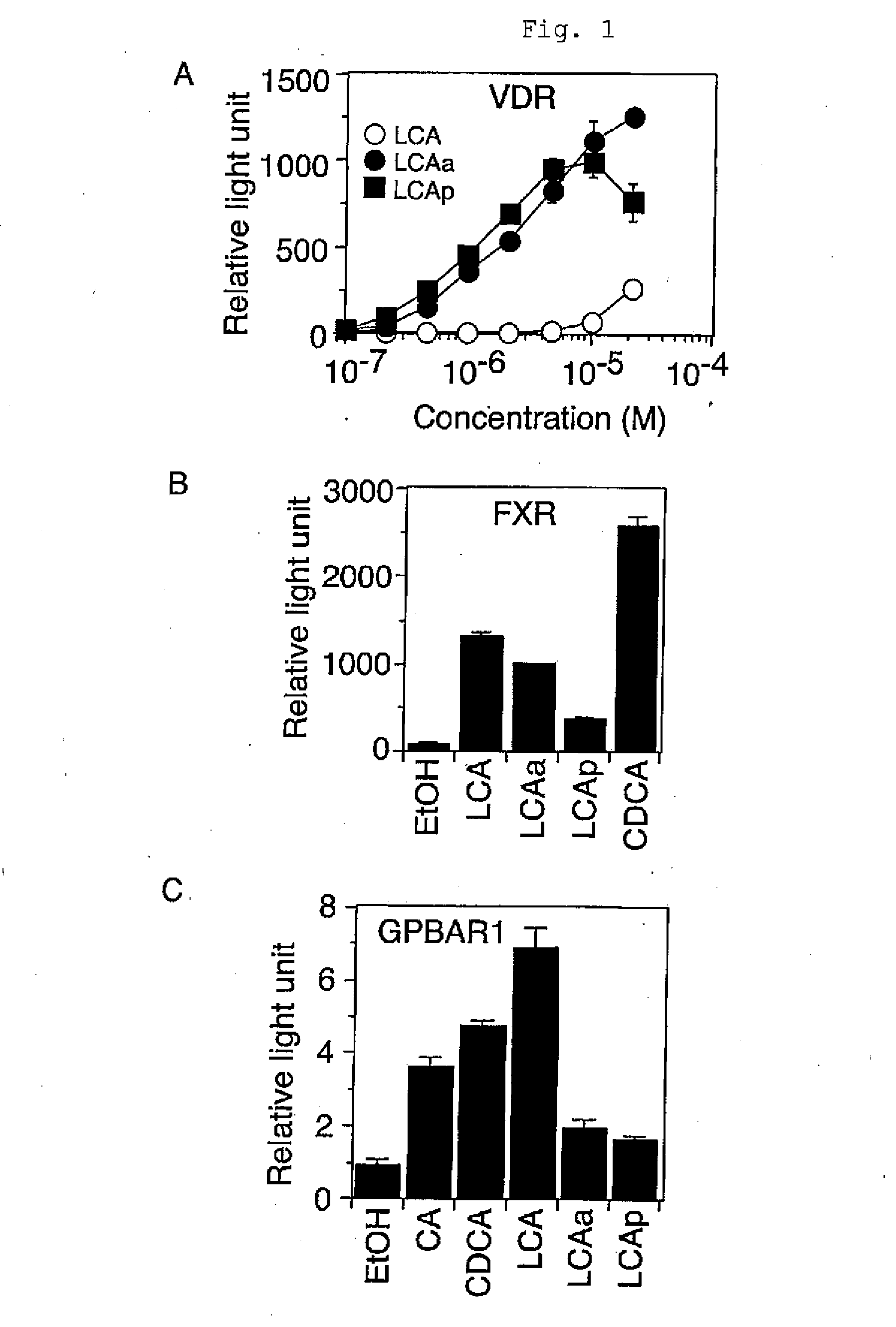
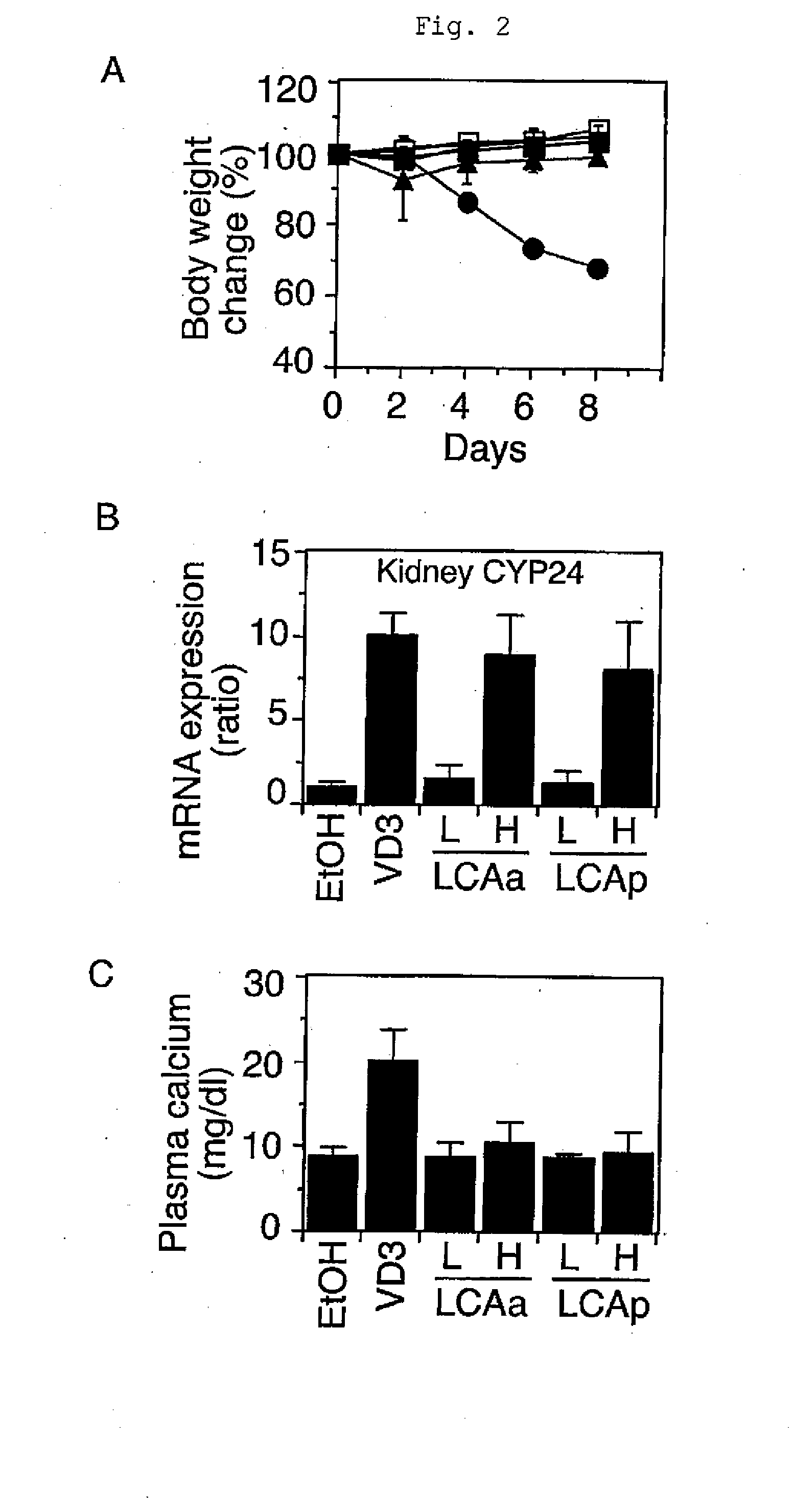
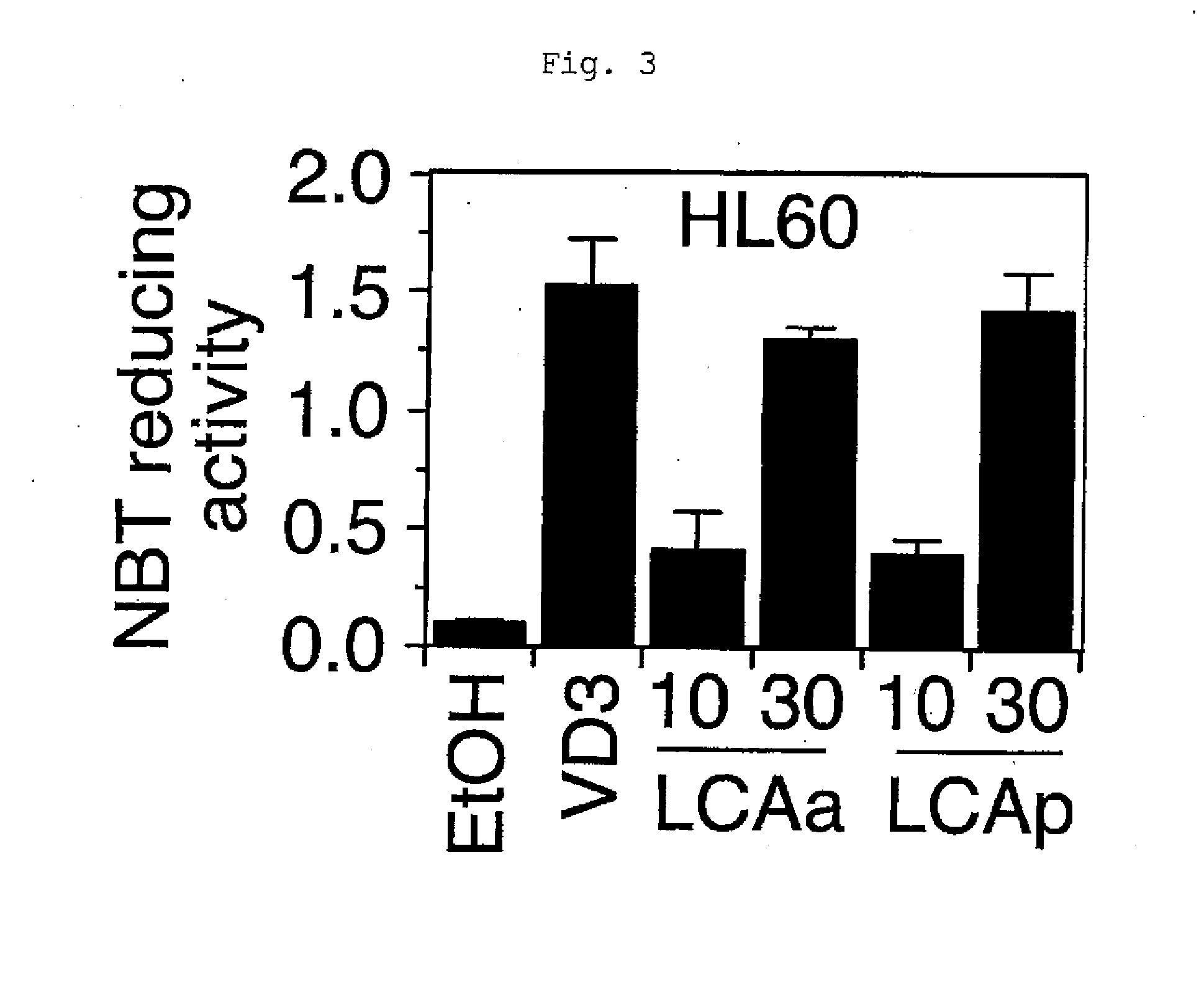
Function-Selective Vitamin D Receptor Agonist
InactiveUS20100204191A1Antibacterial agentsOrganic active ingredientsPropionateFunctional selectivity
The present invention provides a VDR ligand which does not induce hypercalcemia that is an adverse reaction of a vitamin D3 preparation; and a composition which comprises lithocholic acid propionate, a salt thereof, a solvate thereof, or a prodrug thereof.
Owner:NIHON UNIVERSITY
Application of deoxyschizandrin in preparation of drug for treating cholestasis liver injury
The invention discloses an application of deoxyschizandrin in preparation of drug for treating cholestasis liver injury. Deoxyschizandrin has molecular formula of C24H32O6 and molecular weight of 416.51, and has the structural formula shown in the description. Mouse liver injury induced by application of excessive lithocholic acid (LCA) is taken as a model. After application of deoxyschizandrin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in serum are significantly lowered, while necrosis degree of liver cells is significantly improved. The results indicate that deoxyschizandrin has treatment effect for liver injury induced by LCA, and can be used for treating cholestasis liver injury clinically.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
7α-hydroxysteroid dehydrogenase gene s1-a-1
ActiveCN106701707BCatalytic carbonyl asymmetric reduction reactionBacteriaOxidoreductasesCholic acidChenodeoxycholic acid
The invention relates to HSDH (hydroxysteroid dehydrogenase), in particular to a gene S1-a-1 of novel 7 alpha-HSDH. A nucleotide sequence of the gene is shown as SEQ ID NO.2, the novel 7 alpha-HSDH is encoded, a nucleotide sequence of the novel 7 alpha-HSDH is shown as SEQ ID NO.1, the novel 7 alpha-HSDH can be used for catalyzing CDCA (chenodeoxycholic acid) and TCDCA (taurochenodeoxycholic acid) to generate 7K-LCA (7-ketone lithocholic acid) and T7K-LCA (taurine-7-ketone lithocholic acid), wherein the catalytic activity of the novel 7 alpha-HSDH for CDCA is about 5 times of that of 7 alpha-HSDH of Sardinia clostridium, and the catalytic activity of the novel 7 alpha-HSDH for TCDCA is about over 2.5 times of that of 7 alpha-HSDH of Sardinia clostridium, therefore, the novel 7 alpha-HSDH has a great industrial application value.
Owner:CHONGQING KINBEAR BIOTECHNOLOGY CO LTD
Application of lithocholic acid serving as active ingredient in preparation of echinococcosis granulosis cyst treatment medicine
The invention discloses application of lithocholic acid serving as an active ingredient in preparation of echinococcosis granulosis cyst treatment medicine. The echinococcosis granulosis cyst treatment medicine can kill pathogeny-echinococcosis granulosis cyst tapeworms of echinococcosis granulosis cyst through lithocholic acid, has the remarkable restraining and killing effects, and can be used for treating echinococcosis granulosis cyst.
Owner:李思源
Application of monomeric compounds of fruits of Chinese magnoliavine (Schisandra chinensis(Turcz.) baill. and Schisandra sphenanthera Rehd.et Wils) to preparation of medicine for preventing and treating cholestasis liver diseases
InactiveCN107569481APrevention and Treatment of Congestive Liver InjuryProve the effect of the treatmentDigestive systemEther/acetal active ingredientsAlanine aminotransferaseHepatica
The invention discloses application of monomeric compounds of fruits of Chinese magnoliavine (Schisandra chinensis(Turcz.) baill. and Schisandra sphenanthera Rehd.et Wils) to preparation of medicine for preventing and treating cholestasis liver diseases. The monomeric compounds of fruits of Chinese magnoliavine refer to Schisandrin B, Schisandrin C, Schisandrol A, Schisantherrin A and Schisantherrin B. The result of the study shows that all of the five kinds of monomeric compounds can obviously reduce the content of total bile acids and direct bilirubin in blood serum in the cholestasis liverdisease state, can relieve the cholestasis symptoms, can reduce the activity of alkaline phosphatase, gamma-glutamyltransferase, aspartate transaminase and / or alanine aminotransferase in the liver, and can effectively improve the liver tissue necrosis degree. The invention discovers the condition for the first time that the five kinds of monomeric compounds of fruits of Chinese magnoliavine can beused for effectively preventing and treating the cholestasis liver diseases induced by liithocholic acid. The discovery provides the powerful technical theoretical basis for the new medicine researchand development for the cholestasis liver diseases and the clinic application of the monomeric compounds of fruits of Chinese magnoliavine; and wide application prospects are realized in the field ofcholestasis prevention and treatment.
Owner:SUN YAT SEN UNIV
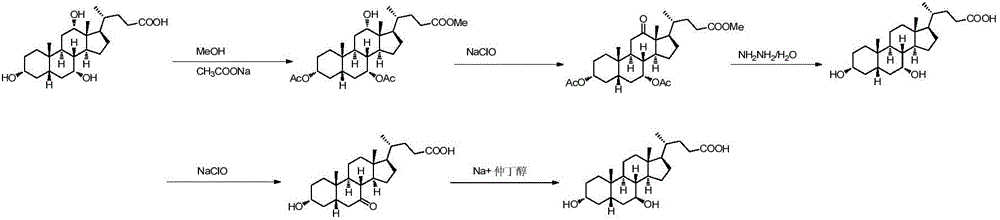
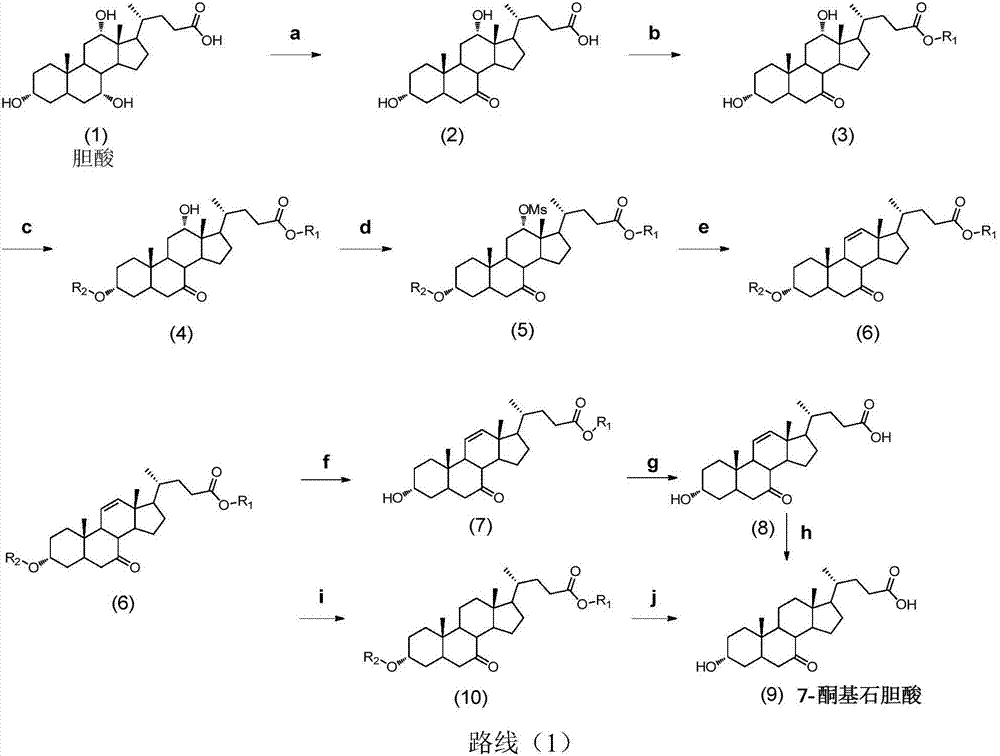
Preparation method of lithocholic acid and intermediates thereof
The invention discloses a synthesis method of lithocholic acid and an intermediates thereof. According to the preparation method of the lithocholic acid intermediate, a compound I reacts with hydrogento generate a compound II in a mixed solvent by taking palladium on carbon as a catalyst and adding specific alkali; a low-price botanical bulk fermentation product BA is used as a raw material, andlithocholic acid is synthesized through side chain construction, hydrogenation, reduction, hydrolysis and other reactions; and the selectivity of 5beta hydrogen in the hydrogenation reaction is improved, high-toxicity reagents such as hydrazine hydrate are prevented from being used for hydroxyl due to removal of other animal-derived cholic acids, and the method is environmentally friendly, high insafety, simple in route, mild in reaction condition and suitable for industrial mass production.
Owner:HUNAN KEREY BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com