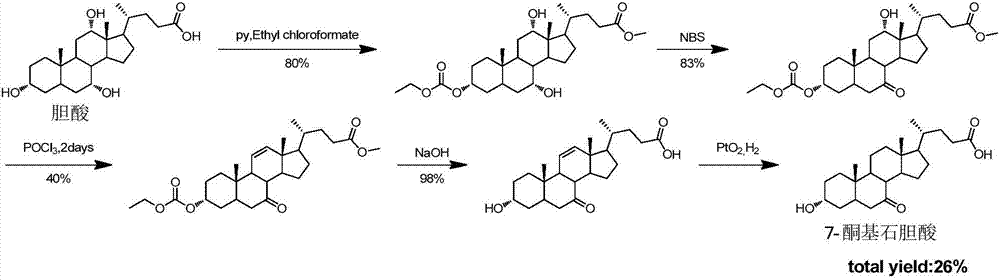
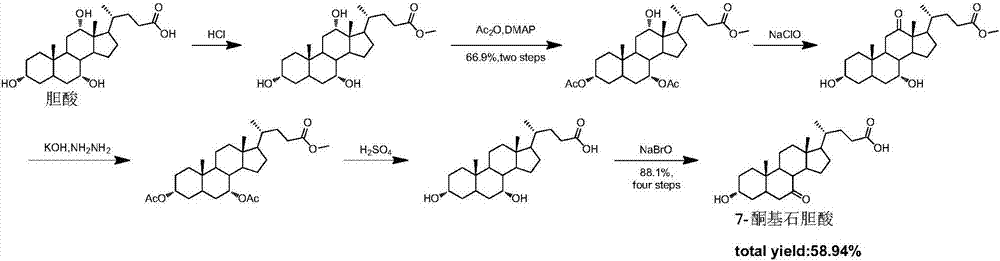
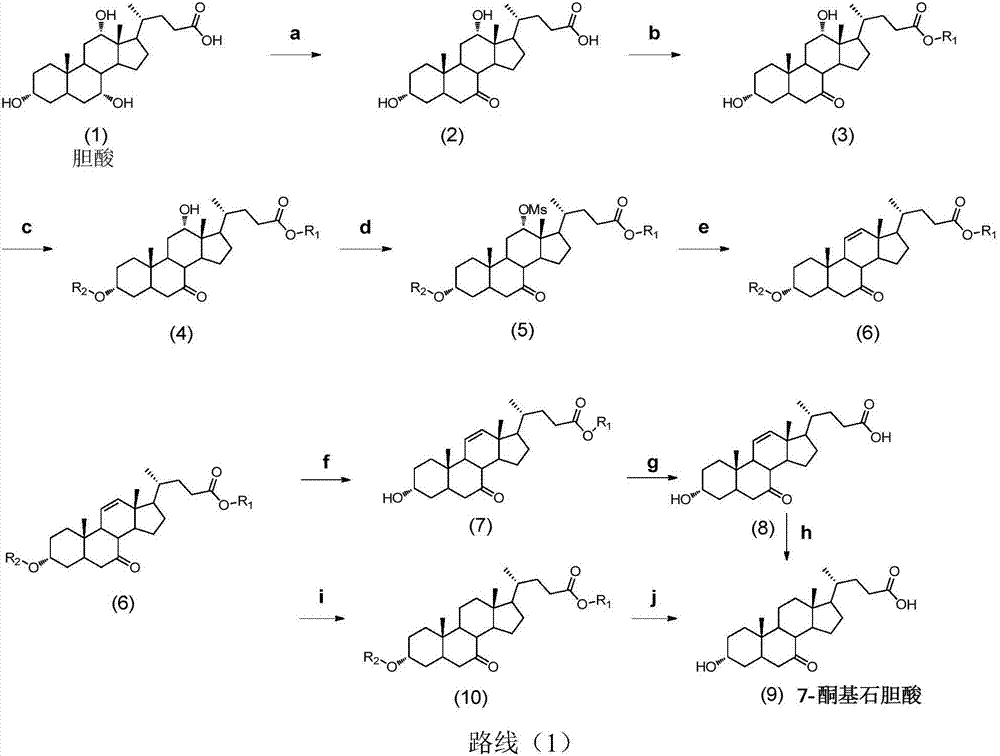
Synthetic method of 7-keto-lithocholic acid
A cholic acid and cornerstone technology, applied in the field of organic chemical synthesis, can solve problems such as low yield, high equipment requirements, and unsuitability for large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111]
[0112] 1. Synthesis of the compound of formula (2): Dissolve cholic acid (9g, 22.0mmol) in 200mL of acetone / water (v / v=3:1), protect from light, slowly add NBS (5.7g, 31.9mmol), Reaction at room temperature 25°C for 2h. After TLC detects that the reaction is complete, add 100mL saturated sodium bisulfite solution to quench the reaction, remove the solvent under reduced pressure until a white solid appears, stop when poured into 1L of water, and precipitate a large amount of white solid, stand for crystallization, filter and dry to obtain Formula (2) compound (8.5g white solid, yield 95%). used directly in the next step.
[0113]
[0114] 2. Synthesis of the compound of formula (3-1): Dissolve the compound of formula (2) (8.5g, 20.8mmol) in 100mL of methanol, add 1mL of concentrated sulfuric acid dropwise, heat to reflux for 2h, remove methanol under reduced pressure, add 30mL of water , the aqueous phase was extracted with dichloromethane (30 mL×3). Combine t...
Embodiment 2
[0128]
[0129] 1. Synthesis of the compound of formula (2): Dissolve cholic acid (9g, 22.0mmol) in 200mL acetone / water (v / v=3:1), stir in the dark, slowly add NBS (5.7g, 31.9mmol) , room temperature 25 ℃ reaction 2h. After TLC detects that the reaction is complete, add 100 mL of saturated sodium bisulfite solution to quench the reaction, concentrate under reduced pressure until a white solid appears, pour it into 1L of water, and precipitate a large amount of white solid, leave it to crystallize, filter and dry to obtain the formula ( 2) Compound (8.5g white solid, yield 95%). used directly in the next step.
[0130]
[0131] 2. Synthesis of the compound of formula (3-1): Dissolve the compound of formula (2) (8.5g, 20.8mmol) in 100mL of methanol, add 1mL of concentrated sulfuric acid dropwise, heat to reflux for 2h, remove methanol under reduced pressure, add 30mL of water , the aqueous phase was extracted with dichloromethane (30 mL×3). Combine the organic phases, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com