Method for preparing amphiphilic chitosan nanometer medicament carrier
A nano- and amphiphilic technology of chitosan, applied in pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve problems such as unfavorable controlled release, poor stability of polymer micelles, and influence on the properties of chitosan, and achieve The effect of good particle size distribution, controllable yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) At room temperature, 1g chitosan M v64KDa was dissolved in 100 mL of aqueous hydrochloric acid with a molar concentration of 0.05, and then the pH of the solution was adjusted to 4 with 1N NaOH to form a transparent 1% (w / v) chitosan solution.
[0031] 2) Get a certain amount of lithocholic acid, make the ratio of the amount of substance of lithocholic acid to the amount of substance of glucosamine residue in chitosan be 0.14: 1, add it in the DMSO solution of 100mL, add to lithocholic acid subsequently EDC and NHS are added into the solution in equal quantities, and the ratio of EDC to lithocholic acid is kept constant at 1.2:1. Lithocholic acid, EDC and NHS were reacted at room temperature at 500rpm for 90min.
[0032] 3) A mixed solution of lithocholic acid, EDC and NHS reacted in 100 mL of DMSO for 90 min was slowly added dropwise to a 1% (w / v) chitosan solution within 30 min, and reacted for 24 h at a stirring speed of 1000 rpm.
[0033] 4) Add the above 200mL...
Embodiment 2
[0037] Except that the molar ratio of lithocholic acid and glucosamine residues in chitosan is 0.24:1, other conditions are the same as in Example 1.
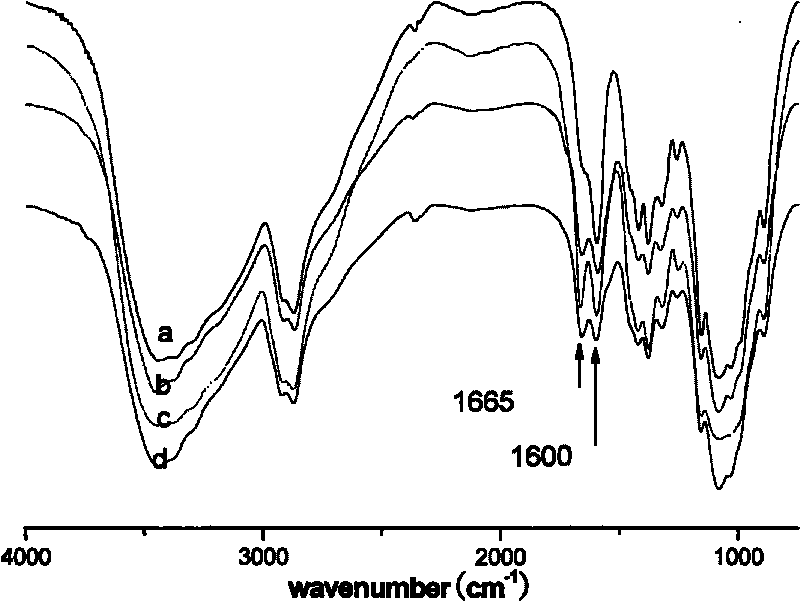
[0038] The FTIR characterization spectrum of the synthesized product under this condition is shown in figure 1 -c.
[0039] The particle size intensity distribution diagram of the nanoparticles prepared under this condition is shown in Figure 4 (Z-average=249.2±27.4 nm).
[0040] The TME morphology characterization diagram of the nanoparticles prepared under this condition is shown in Figure 5 .
Embodiment 3
[0042] Except that the molar ratio of lithocholic acid and glucosamine residue in chitosan is 0.34: 1, other conditions are the same as specific example 1.
[0043] The FTIR characterization spectrum of the synthesized product under this condition is shown in figure 1 -d.
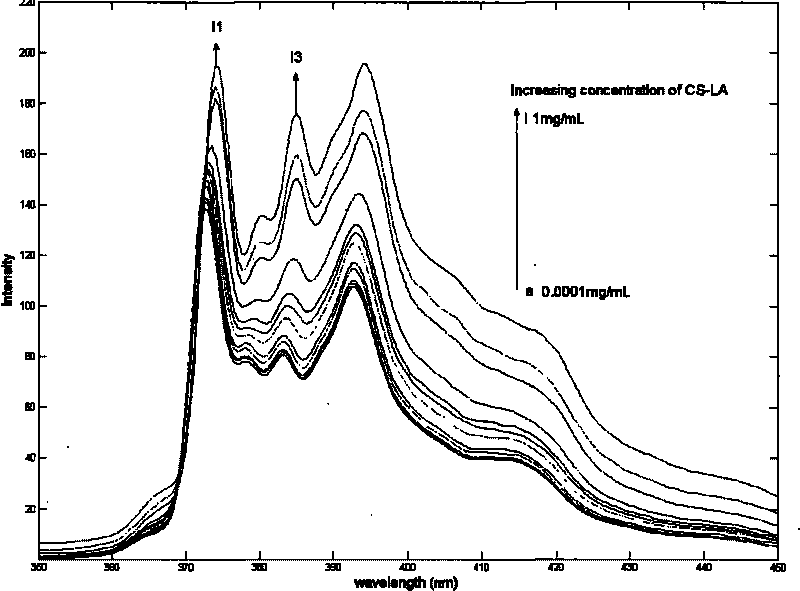
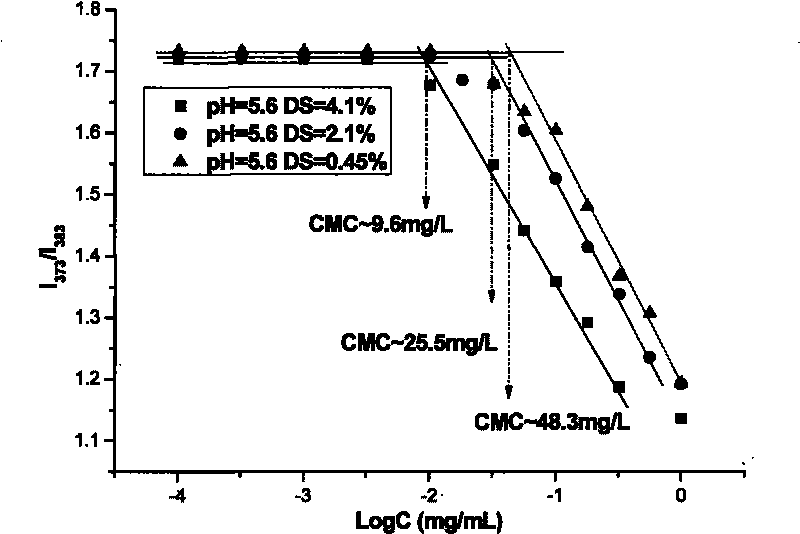
[0044] When the synthetic product under this condition is characterized by pyrene molecular fluorescent probe to characterize the critical micelle concentration, the obtained pyrene emission spectrum is shown in figure 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com