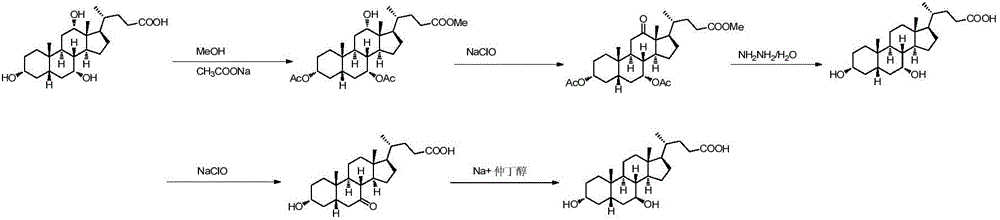
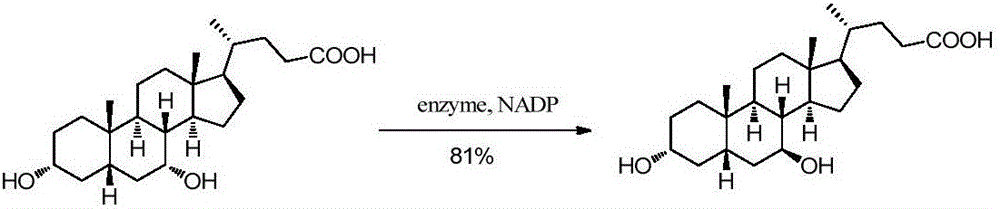
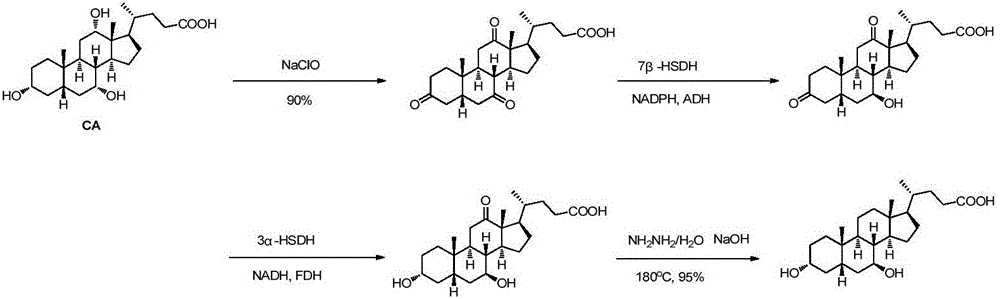
Chemical-enzymatic preparation of UDCA
A technology for the preparation of ursodeoxycholic acid and enzymatic method, which is applied in the field of chemical-enzymatic preparation of ursodeoxycholic acid, can solve the problems of difficult industrial application, stay in the laboratory research stage, low substrate concentration, etc., and achieve the reaction The effect of mild conditions, cheap raw materials, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Synthesis of ursodeoxycholic acid (compound I)
[0039] (1) Preparation of 12-ketodeoxycholic acid (compound III)
[0040] Add 50mM potassium phosphate buffer (10mL, pH=8.0) into a 30mL reaction vessel, add compound II (1.5g), NADP (1.5mg), acetone (1.5mL), stir well and then add 12α-HSDH101 enzyme powder ( 0.15 g) and alcohol dehydrogenase KRED195 (30 mg) were adjusted to 15 mL, and reacted with magnetic stirring at 35°C for 24 hours. After the reaction, heat at 70°C for 2 hours to denature the protein in the reaction solution, remove the protein by filtration, adjust the pH to 3 with 1M hydrochloric acid, extract three times with an equal volume of ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, and distill under reduced pressure to obtain 12- Ketodeoxycholic acid (compound III, 1.38 g, yield 92%).
[0041] (2) Preparation of gallstone acid (compound IV)
[0042] Add compound III (1.38g), ethylene glycol (10ml), 85% hydrazine...
Embodiment 2
[0045] The synthesis of embodiment two ursodeoxycholic acid (compound I)
[0046] (1) Preparation of 12-ketodeoxycholic acid (compound III)
[0047]Add 60mM potassium phosphate buffer solution (10mL, pH=7.0) into a 50mL reaction vessel, add compound II (1.5g), NADP (1.5mg), acetone (1.5mL), stir well and then add 12α-HSDH101 enzyme powder ( 150mg) and alcohol dehydrogenase KRED195 (30mg) were adjusted to 30mL, and reacted with magnetic stirring at 40°C for 24 hours. After the reaction, heat at 70°C for 2 hours to denature the protein in the reaction solution, remove the protein by filtration, adjust the pH to 3 with 1M hydrochloric acid, extract three times with an equal volume of ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, and distill under reduced pressure to obtain 12- Ketodeoxycholic acid (compound III, 1.43 g, yield 94%).
[0048] (2) Preparation of gallstone acid (compound IV)
[0049] Add compound III (1.43g), ethylene glycol (15ml),...
Embodiment 3
[0052] Example 3 Synthesis of ursodeoxycholic acid (compound I)
[0053] (1) Preparation of 12-ketodeoxycholic acid (compound III)
[0054] Add 40mM potassium phosphate buffer solution (10mL, pH=7.0) into a 100mL reaction vessel, add compound II (1.5g), NADP (1.0mg), acetone (1.5mL), stir well and then add 12α-HSDH101 enzyme powder ( 300mg) and alcohol dehydrogenase KRED195 (50mg) were adjusted to 50mL, and reacted with magnetic stirring at 30°C for 20 hours. After the reaction, heat at 70°C for 2 hours to denature the protein in the reaction solution, remove the protein by filtration, adjust the pH to 3 with 1M hydrochloric acid, extract three times with an equal volume of ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, and distill under reduced pressure to obtain 12- Ketodeoxycholic acid (compound III, 1.34 g, yield 90%).
[0055] (2) Preparation of gallstone acid (compound IV)
[0056] Add compound III (1.34g), ethylene glycol (15ml), 85% hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com