Benzoyl formic acid synthesis method
A technology of benzoylformic acid and a synthesis method, applied in the field of compound synthesis of organic chemistry, can solve the problems of expensive raw materials, serious environmental pollution, long reaction time and the like, and achieve the effects of improving cleanliness, reducing complexity and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
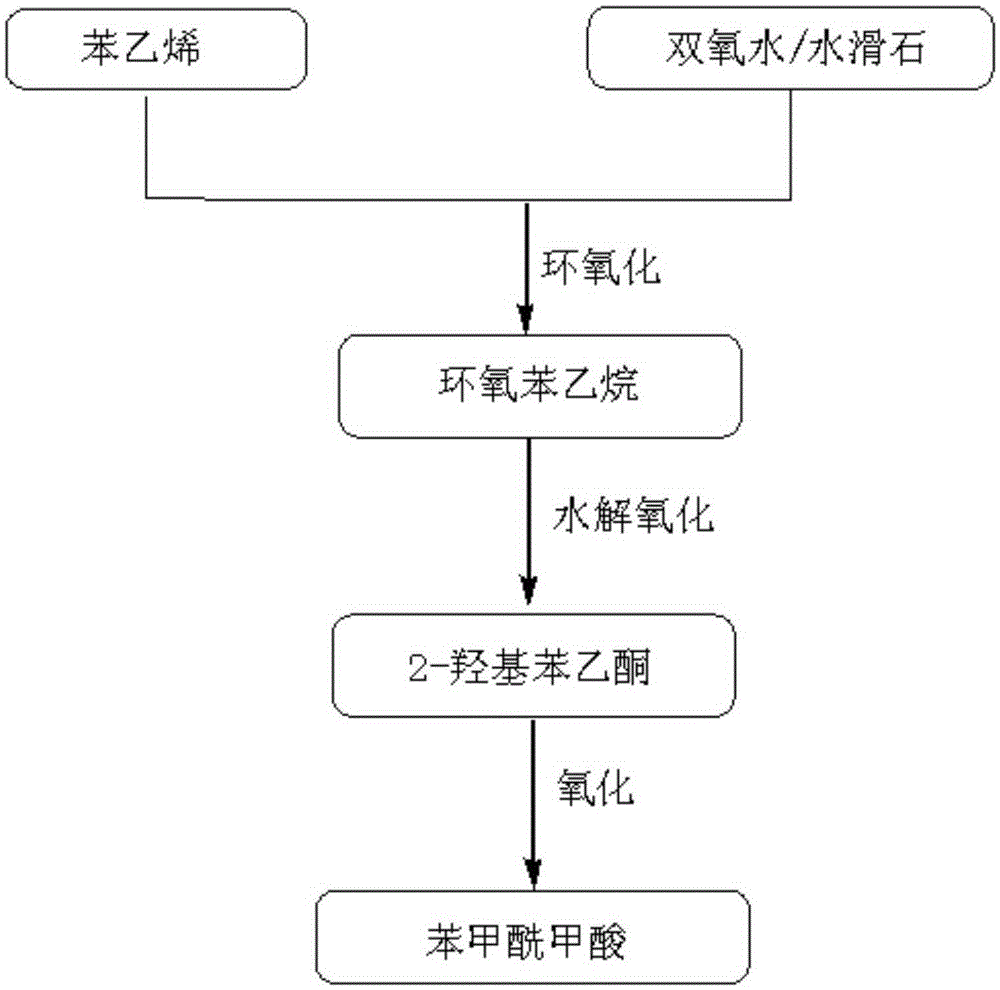
[0036]Pipette 10mmol styrene and add it into a three-necked flask. Add 10mL of acetonitrile, 0.10g of hydrotalcite, and slowly dropwise add 2.4mL of hydrogen peroxide under constant stirring. After the dropwise addition, stir for a while, then slowly heat to 60°C and cool down to room temperature after the reaction. Add 10mL of water, 0.50mmol of cerium ammonium nitrate, and 1.00g of N-bromosuccinimide to the reaction mixture after filtering out the hydrotalcite, control the reaction at 30°C to complete the reaction, and separate α-hydroxyacetophenone by distillation under reduced pressure . In the reactor equipped with α-hydroxyacetophenone, add 10mL of water and 10mmol of 48% hydrobromic acid under constant stirring, and slowly add 80mmol of 30% hydrogen peroxide dropwise. After the addition, slowly raise the temperature to 90°C, and slowly cool down after the reaction To room temperature, the organic phase and the aqueous phase are separated by standing, and the separated ...
Embodiment 2
[0038] Pipette 10 mmol styrene into the reactor. Add 10mL of acetonitrile and 0.10g of hydrotalcite under continuous stirring, and slowly add 2.5mL of hydrogen peroxide dropwise. After the dropwise addition, slowly raise the temperature to 60°C to complete the reaction. After filtering out the mixed solution of hydrotalcite, add 5 mL of water, 0.5 mmol of cerium ammonium nitrate, and 1.00 g of N-bromosuccinimide under stirring, control the temperature at 40°C to complete the reaction, and distill α-hydroxyacetophenone under reduced pressure. Add it into a reactor with 10mL of water, add 10mmol of 48% hydrobromic acid with stirring, then slowly add 100mmol of 30% hydrogen peroxide dropwise at room temperature, after the addition, slowly raise the temperature to 90°C to complete the reaction. The organic phase was separated at room temperature, distilled, purified and dried. The total yield of benzoylformic acid was 71.3%, and the HPLC purity was 98.7%.
Embodiment 3
[0040] Pipette 10mmol of styrene into the reactor, add 10mL of n-hexane and 0.10g of hydrotalcite successively under continuous stirring, slowly add 2.4mL of hydrogen peroxide dropwise, after the dropwise addition, slowly heat up to 60°C and react until the styrene is completely converted , cooled to room temperature, and after the hydrotalcite was separated, 10mL of acetonitrile and water mixed solution, 0.6mmol of cerium ammonium nitrate, 1.00g of N-bromosuccinimide were added successively, and the reaction was completed at 50°C, and α- Hydroxyacetophenone. Add the separated α-hydroxyacetophenone into a reactor with 10mL of water, add 10mmol 48% hydrobromic acid under continuous stirring, and then slowly add 100mmol 30% hydrogen peroxide dropwise, and then slowly raise the temperature until the reflux reaction ends. The organic phase was separated at room temperature, and after distillation, purification and drying, the total yield of benzoylformic acid was 79.3%, and the HP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com