Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Azacitidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
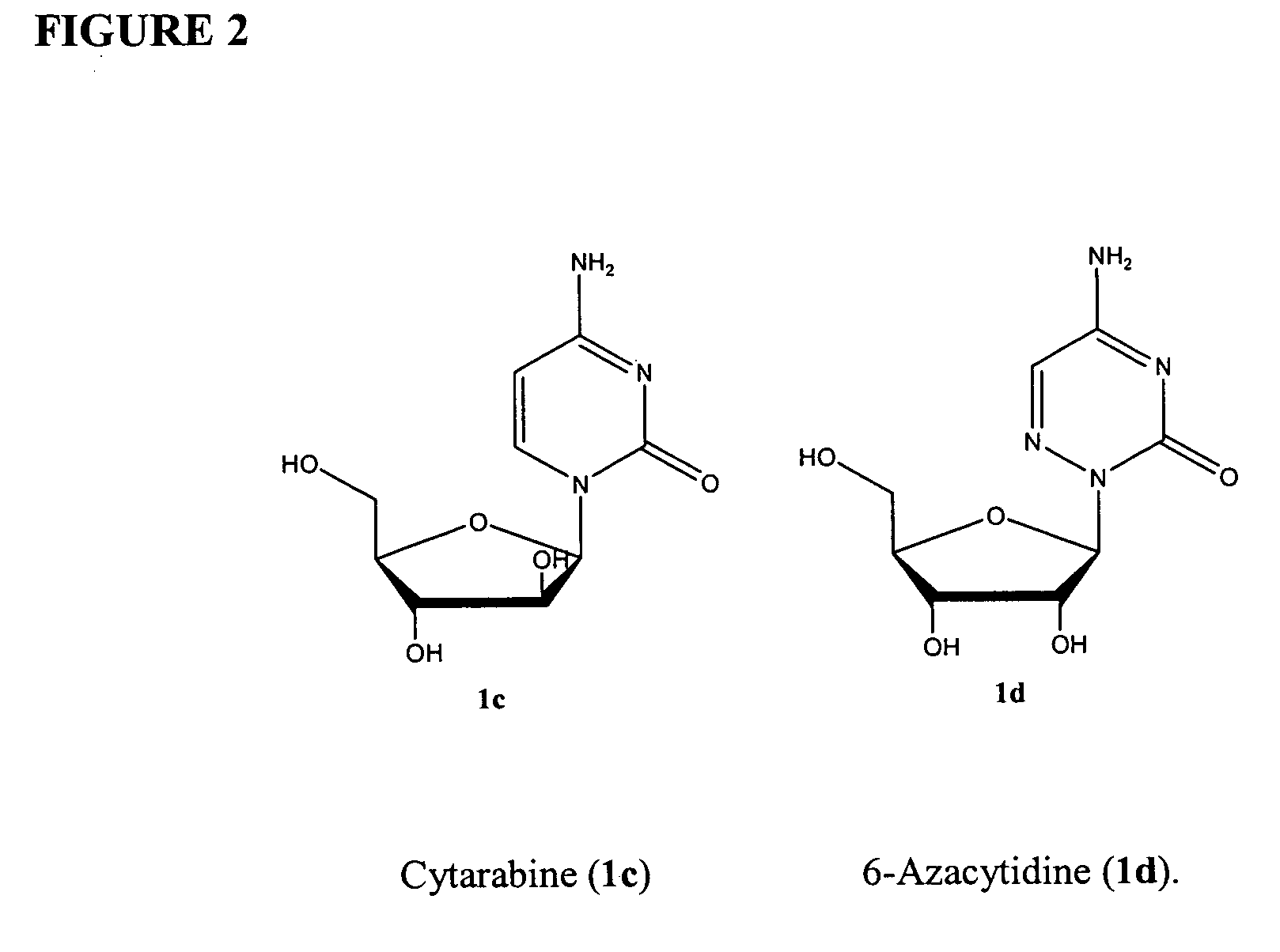
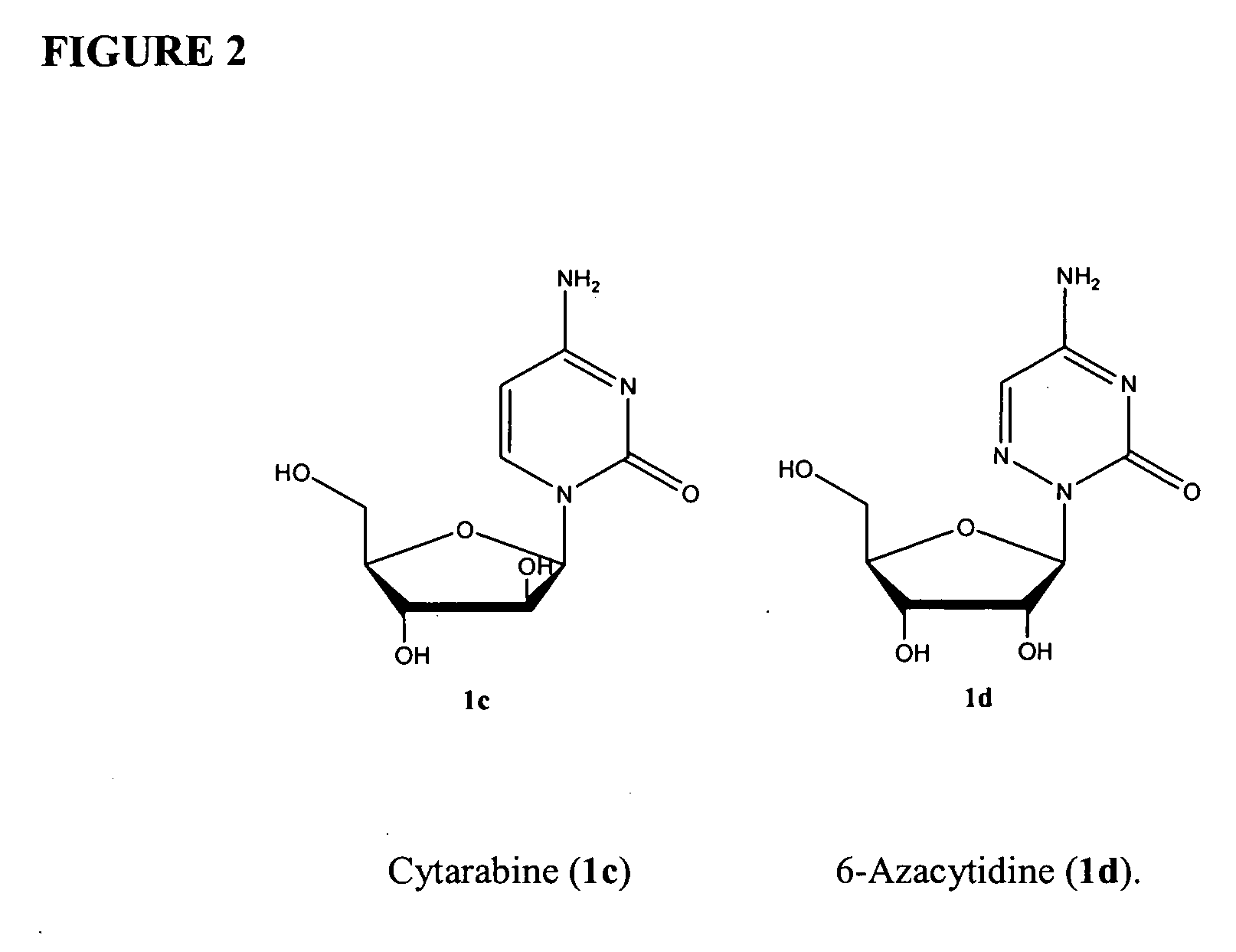
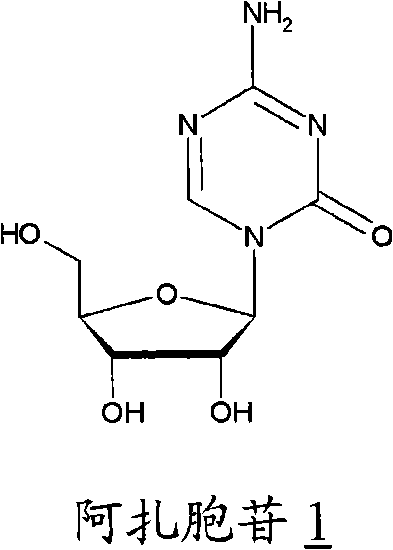
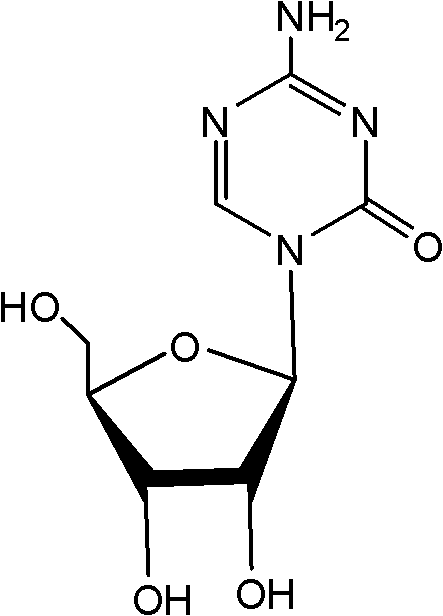
This medication is used to treat a group of blood/bone marrow disorders (myelodysplastic syndromes-MDS) in which the bone marrow does not produce enough healthy blood cells.
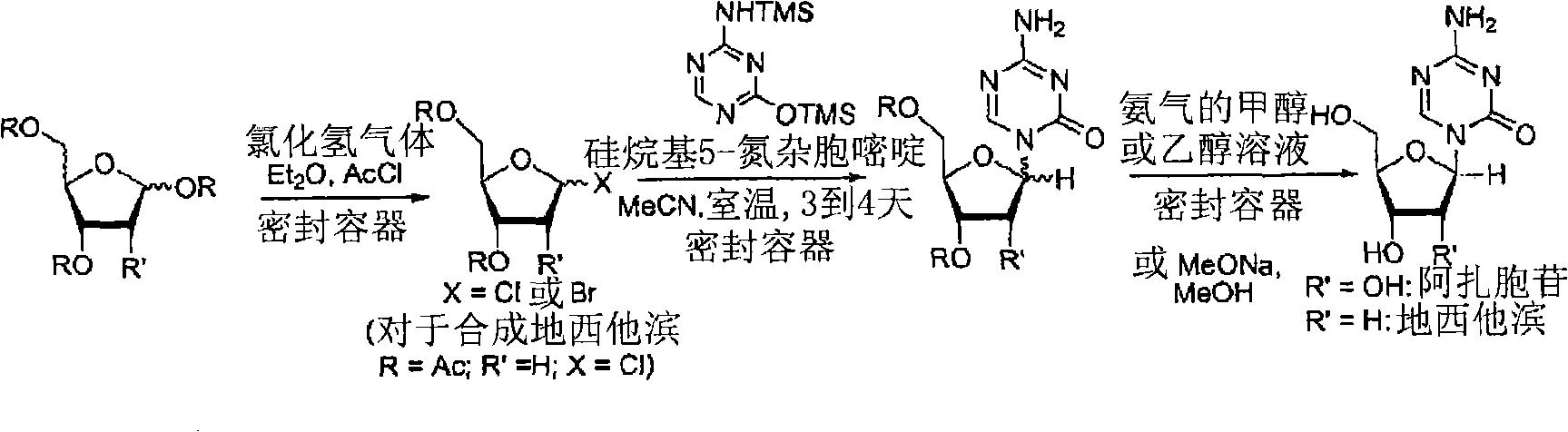
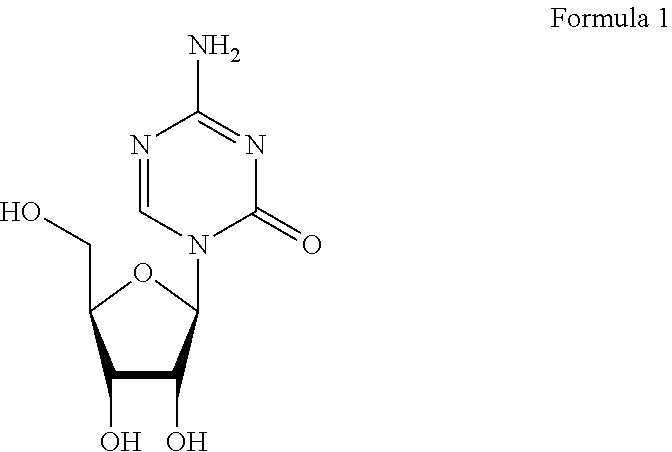
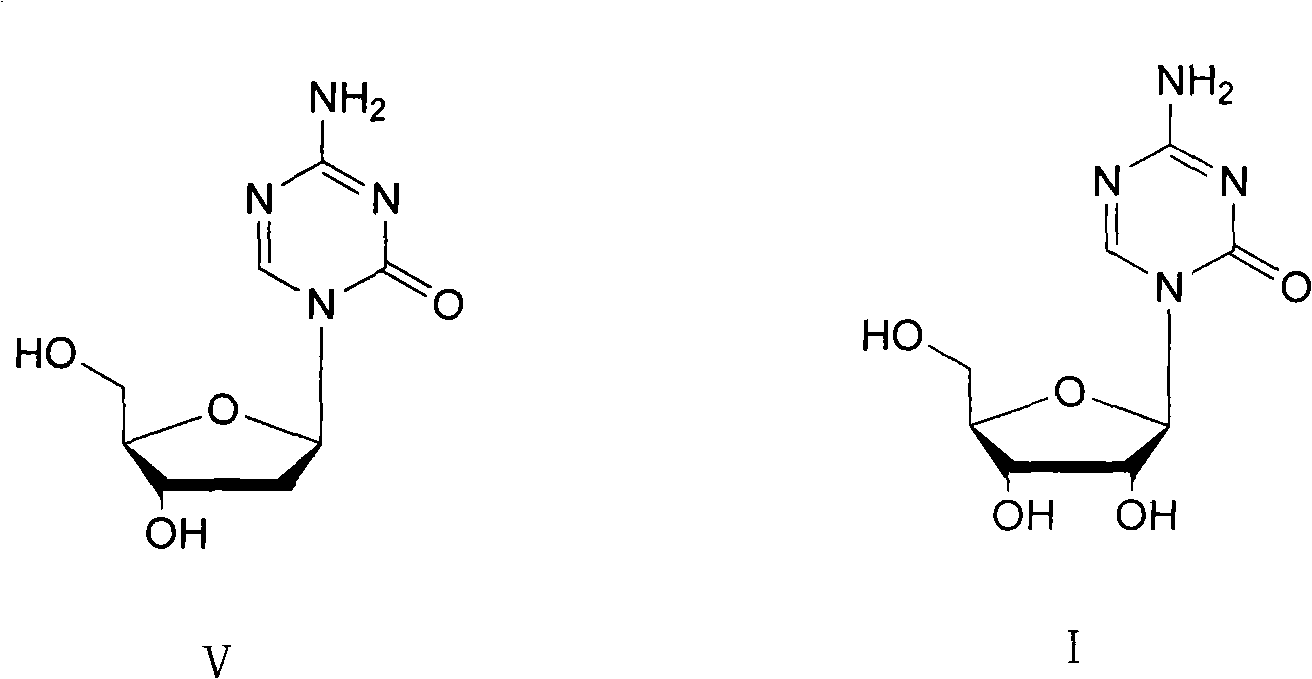
Azacytosine analogs and derivatives
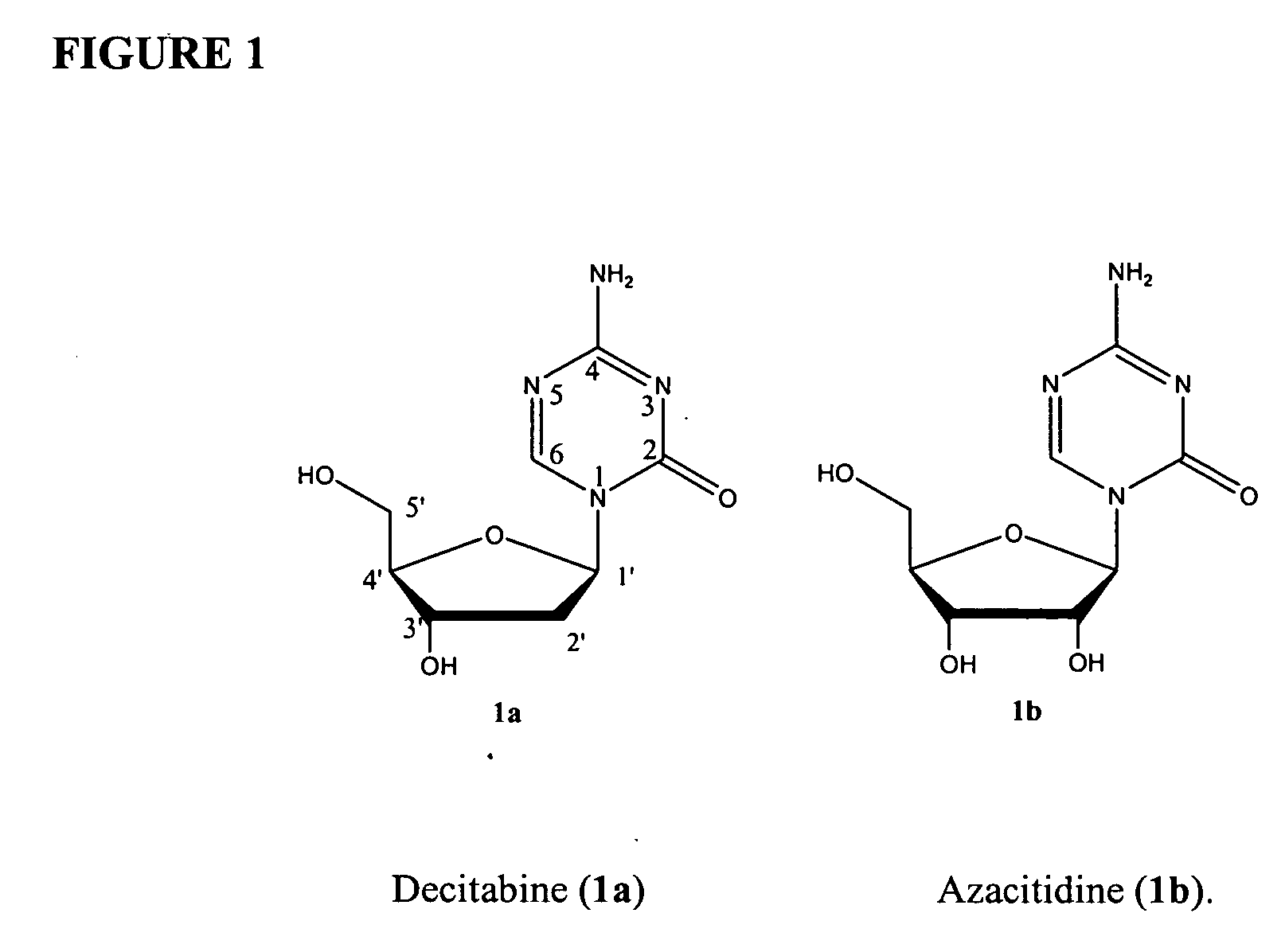
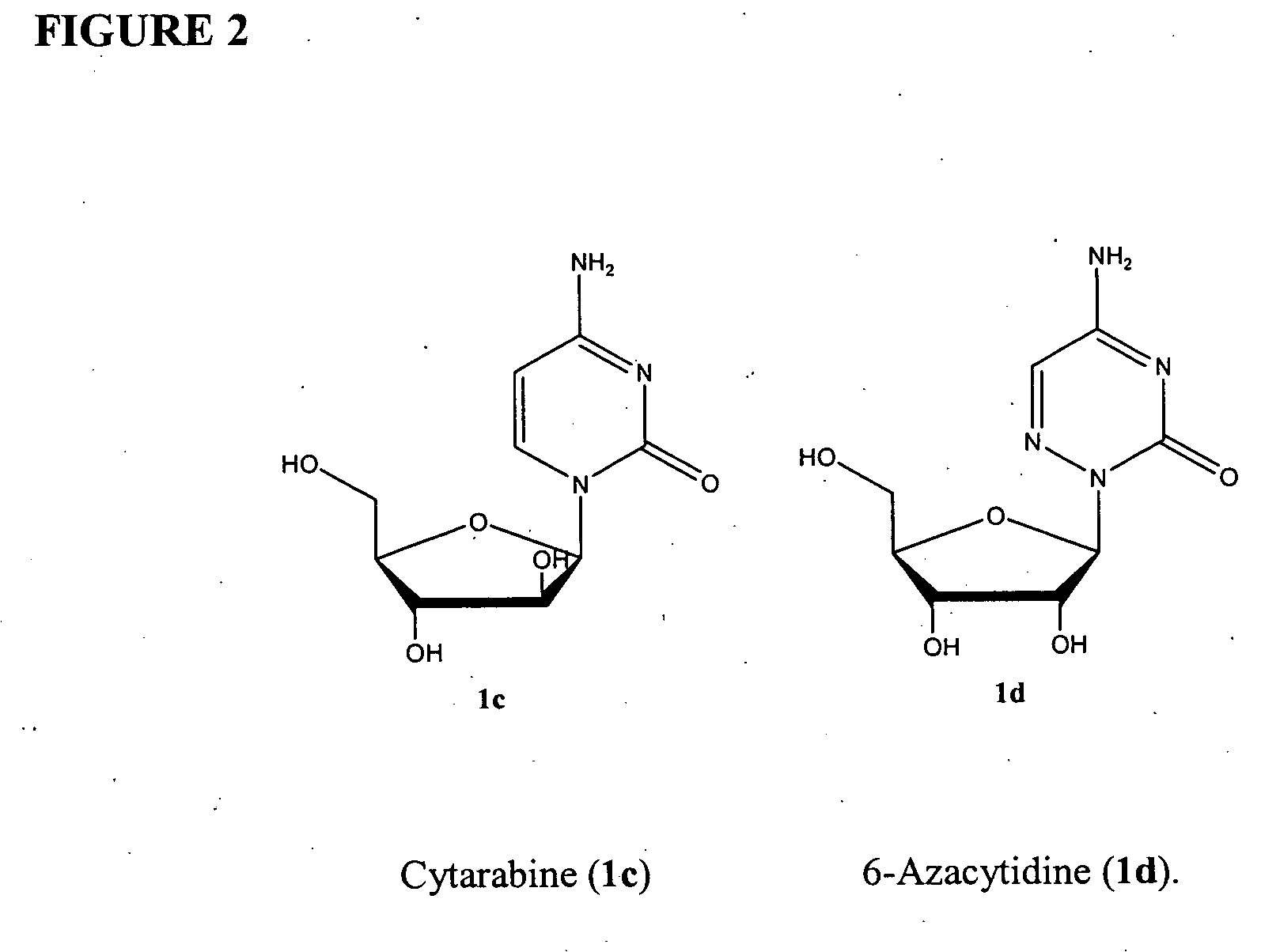
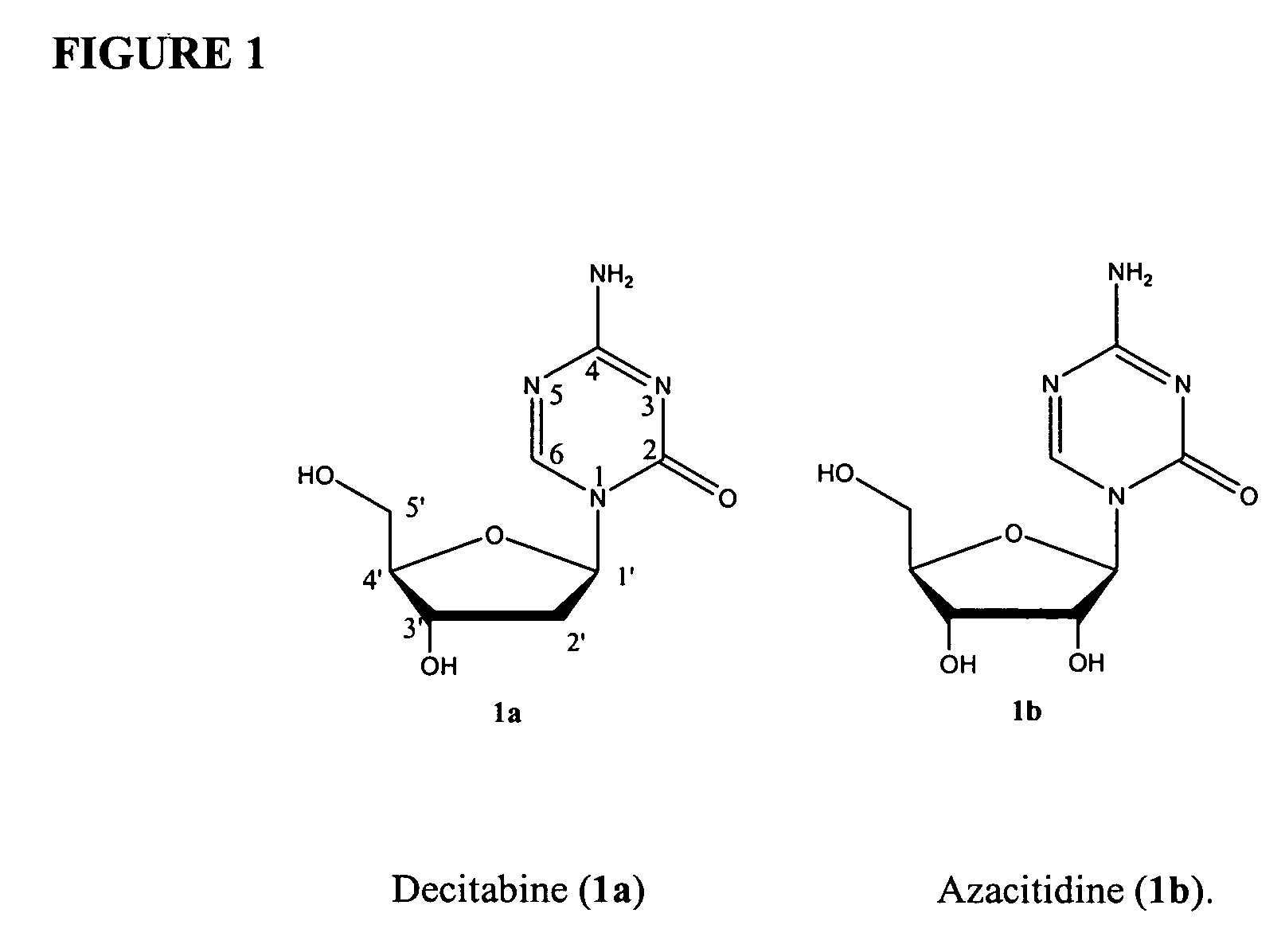
Compounds and compositions of azacytosine analogs and derivatives are provided. In one aspect of the invention, analogs or derivatives of decitabine and azacitidine are provided with modification at the 4- and 6-position of the triazine ring, at the 1′-6′position of the ribose ring, or combinations thereof. Methods of synthesizing and manufacturing these analogs and derivatives are also provided. These compounds can be formulated into pharmaceutical compositions that can be used for treating any disease that is sensitive to the treatment with decitabine or azacitidine, such as hematological disorders and cancer.
Owner:SUPERGEN
Formulations of azacitidine and its derivatives
The present invention relates to pharmaceutical formulations comprising azacitidine or its pharmaceutically acceptable salts, including processes for preparing the formulations comprising azacitidine, or salts thereof, and methods of using the formulations for treating various cancer disorders in mammals.
Owner:DR REDDYS LAB LTD +1
Azacytosine analogs and derivatives
Compounds and compositions of azacytosine analogs and derivatives are provided. In one aspect of the invention, analogs or derivatives of decitabine and azacitidine are provided with modification at the 4- and 6-position of the triazine ring, at the 1′–6′ position of the ribose ring, or combinations thereof. Methods of synthesizing and manufacturing these analogs and derivatives are also provided. These compounds can be formulated into pharmaceutical compositions that can be used for treating any disease that is sensitive to the treatment with decitabine or azacitidine, such as hematological disorders and cancer.
Owner:SUPERGEN
Azacytosine analogs and derivatives
InactiveUS20060205687A1Decrease electrophilicityBiocideSugar derivativesAbnormal tissue growthPyrimidine analogue
Compounds and compositions of azacytosine analogs and derivatives are provided. In one aspect of the invention, analogs or derivatives of decitabine and azacitidine are provided with modification at the 2-, 4-, or 6-position of the triazine ring, at the 1′-6′position of the ribose ring, or combinations thereof. Methods of using, synthesizing and manufacturing these analogs and derivatives are also provided. These compounds can be formulated into pharmaceutical compositions that can be used for treating any disease associated with aberrant DNA methylation, or a disease or condition that is sensitive to the treatment with decitabine or azacitidine, such as hematological disorders, tumors and cancers.
Owner:SUPERGEN
Process for Making 5-Azacytosine Nucleosides and Their Derivatives
ActiveUS20100036112A1High boiling pointIncrease polarityEsterified saccharide compoundsOrganic active ingredientsDecitabineSilylation
A process of synthesizing a 5-azacytosine nucleoside, such as azacitidine and decitabine, comprises coupling a silylated 5-azacytosine with a protected D-ribofuranose of formula in the presence of a sulfonic acid catalyst.
Owner:SCINOPHARM TAIWAN LTD
Azacitidine for injection and preparation method thereof
InactiveCN103251564AInhibit rapid hydrolysisAvoid hydrolysisPowder deliveryOrganic active ingredientsFreeze-dryingMannitol
Azacitidine for injection and a preparation method thereof are disclosed. Main components of the azacitidine for injection contain main drugs of azacitidine and mannitol. Mainly by controlling pH value of an azacitidine aqueous solution, hydrolysis of azacitidine is effectively inhibited, related substances of freeze-dried product are minimized, and product quality of the azacitidine freeze-drying preparation is raised.
Owner:FUKANGREN BIO PHARMA
Azacitidine freeze-drying powder injection and preparation method thereof
InactiveCN101632643ANot prone to oxidationLong storage timePowder deliveryOrganic active ingredientsVitamin CMANNITOL/SORBITOL
The invention discloses a medicinal azacitidine freeze-drying powder injection for treating myelodysplastic syndrome and a preparation method thereof. The prescription of the azacitidine freeze-drying powder injection comprises azacitidine, mannitol and vitamin C. The invention solves the problem of rapid impurity increase caused by different crystal forms formed in the processes of rapidly freezing and drying the prior powder injection by optimizing the prescription and improving the preparation method.
Owner:HANGZHOU XIANDA MEDICINE TECH
Process for making 5-azacytosine nucleosides and their derivatives
ActiveCN102216315AShorten the timeSuitable for large-scale synthesisEsterified saccharide compoundsBiocideDecitabine5-azacytosine
A process of synthesizing a 5-azacytosine nucleoside, such as azacitidine and decitabine, comprises coupling a silylated 5-azacytosine with a protected D-ribofuranose of formula in the presence of a sulfonic acid catalyst.
Owner:SCINOPHARM TAIWAN LTD
Azacitidine freeze-dried preparation and preparation method thereof
ActiveCN104706650ASimple preparation processGood repeatabilityOrganic active ingredientsPowder deliveryOrganic solventFreeze-drying
The invention belongs to the field of pharmaceutical preparations and in particular relates to an azacitidine freeze-dried preparation with stable proprieties. The active ingredient of the azacitidine freeze-dried preparation is a treatment effective amount of azacitidine; a solution contains an organic solvent, a freeze-drying proppant and injection water before freeze-drying; the organic solvent is selected from ethanol, isopropanol, methanol or a mixed solution thereof in any ratio, and preferably is ethanol; and the usage amount of the organic solvent is 0.3-6.0% of the total volume of the solution before freeze-drying. The invention also provides a preparation method of the azacitidine freeze-dried preparation which is simple in process, is suitable for scale production and can be clinically used.
Owner:QILU PHARMA HAINAN
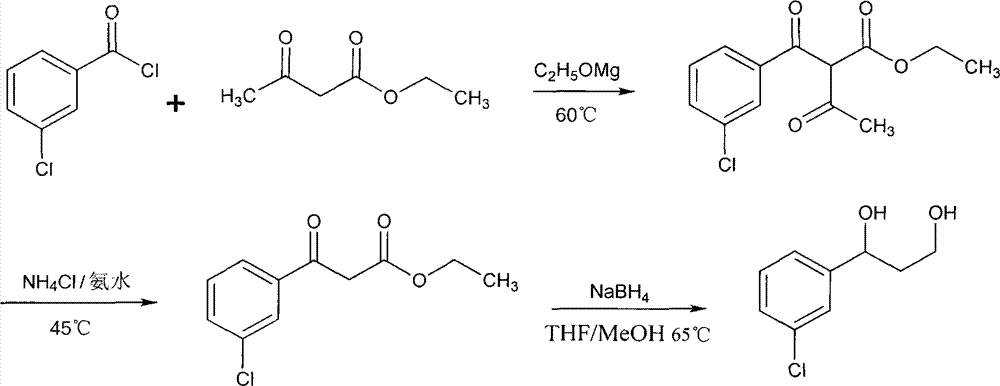
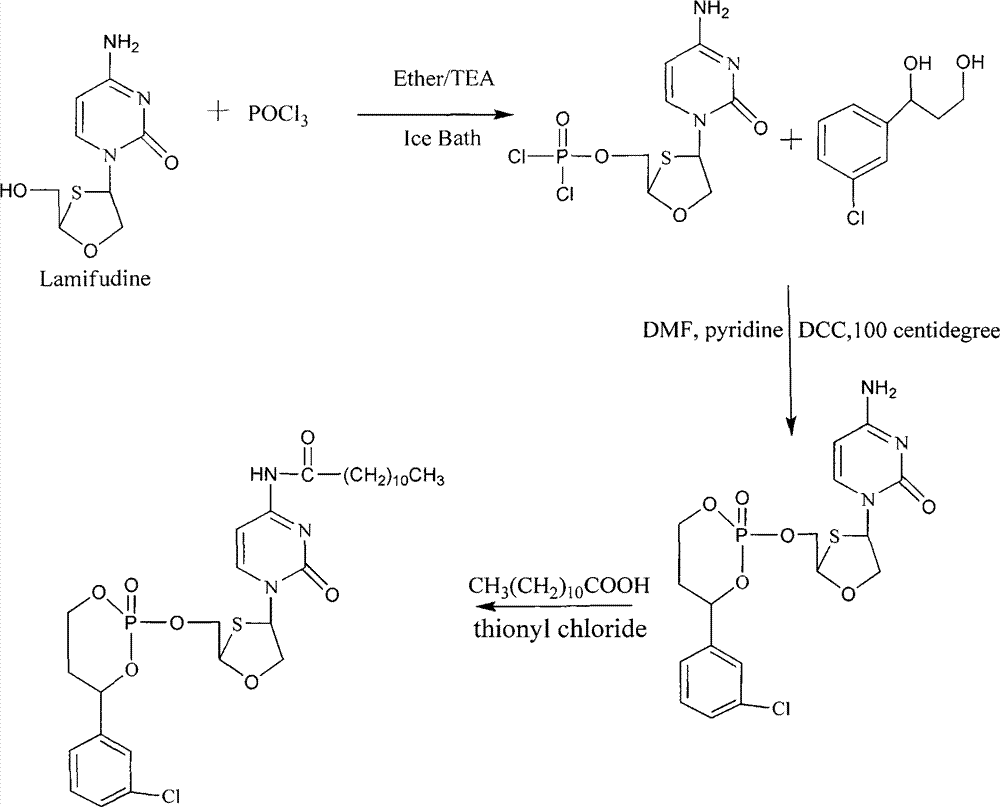
Phosphoryl N-fatty acyl nucleoside analogue for treating viral hepatitis and liver cancer
The invention discloses a phosphoryl N-fatty acyl nucleoside analogue for treating viral hepatitis and liver cancer. The phosphoryl N-fatty acyl nucleoside analogue is characterized in that a nucleoside analogue is modified by a cyclophosphoryl group and then is connected to aliphatic chains having different numbers of carbon atoms. The phosphoryl N-fatty acyl nucleoside analogue can be used for convenient preparation of a nanometer transmission system and has obvious hepatocyte and tumor targeting. The nanometer transmission system comprises liposome, nonionic surfactant niosomes, nanoparticles, nano-emulsion and a self-assembled transmission system. The nucleoside analogue is selected from lamivudine, adenine arabinoside, cidofovir, gemcitabine, cytosine arabinoside, azacitidine and fludarabine. After intravenous administration, the nanometer transmission system of the phosphoryl N-fatty acyl nucleoside analogue has effects of targeting treatment on viral hepatitis and liver cancer.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Method for synthesizing azacitidine
ActiveCN101974051AShorten reaction timeReduce waste volumeSugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsAmmonium compoundsSynthesis methods
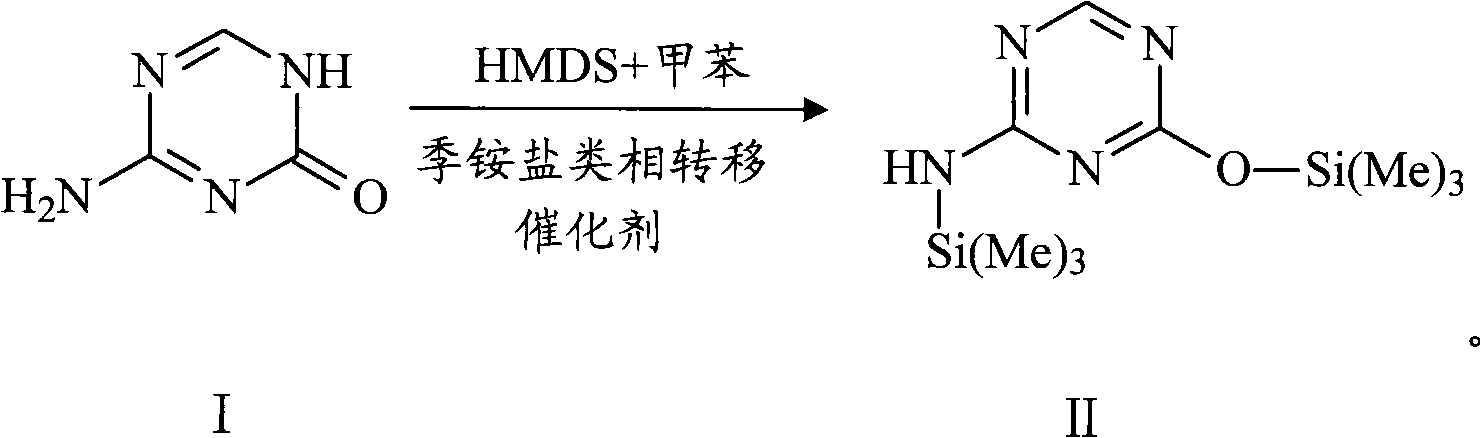
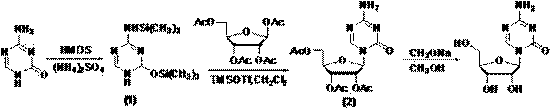
The invention relates to the field of pharmaceutical synthesis process and discloses a method for synthesizing azacitidine. The method comprises the following steps: performing silanization reaction on bis(trimethyl disilylamine) and 5-azacytosine by taking toluene as solvent under the catalysis of phase transfer catalyst of quaternary ammonium compounds under a moisture-isolated condition to generate a compound with structure of formula II; performing condensation reaction between the compound and ribose tetracetate to generate a compound with a structure of formula IV; and performing alcoholysis on the compound in an alkaline environment to generate the azacitidine. The synthesis method of the invention shortens the time of silanization reaction, reduces the waste amount of the bis(trimethyl disilylamine) and avoids influence of the moisture in methanol and ethanol in the purification stage on the purity of the finished product of azacitidine; moreover, the synthesis method has the advantages of cheap reagents, few reaction steps and mild reaction conditions and is favorable for industrial production.
Owner:重庆兴泰濠制药有限公司
Process for the synthesis of azacitidine and decitabine
ActiveCN102206240AHigh yieldAvoid processing powerGroup 4/14 element organic compoundsSugar derivativesOrganic solventTrimethylsilyl
Described herein is a process for the synthesis of azacitidine or decitabine, comprising the silylation of azacytosine in the presence of N,O-bis-trimethylsilyl)-trifluoroacetamide. Such reaction is performed in an organic solvent, preferably aprotic, even more preferably selected from among dichloromethane, dichloroethane and / or acetonitrile. According to a further aspect of the process, 2 to 3 moles of N,O-bis-trimethylsilyl-trifluoroacetamide are used per mole of azacytosine, preferably from 2.2 to 2.5.
Owner:CHEMI SPA
Preparation method of azacitidine
ActiveCN102702292AReduce usageReduce generationSugar derivativesSugar derivatives preparationChemical industryPharmaceutical industry
The invention relates to the fields of chemical industry and chemical pharmaceutical industry and particularly relates to a preparation method of azacitidine. The preparation method disclosed by the invention comprises the steps of: preparing compound I, then uniformly mixing the compound I and 1,2,3,5-Tetra-O-Acetyl-D-Ribose, vacuumizing, controlling the vacuum degree at -0.001-0.95MPa, fusing the uniformly mixed compound I and the 1,2,3,5-Tetra-O-Acetyl-D-Ribose at 145-190 DEG C, cooling the mixture to obtain compound II after reacting for 1.5-4.5 hours, and carrying out alcoholysis on the compound II to prepare the azacitidine. According to the method, the usage amount of the solvent can be reduced, the side reaction and generation of impurities are reduced, and the reaction efficiency is improved.
Owner:HUZHOU ZHANWANG PHARMA
Synthetic method of azacitidine
InactiveCN103524584AAvoid processing powerProcess raw materials are easy to getSugar derivativesSugar derivatives preparationTriflic acidAzacitidine
The invention discloses a synthetic method of azacitidine. The synthetic method comprises the following steps: with 5-azacytosine and tetraacetylribose as raw materials, protecting the raw materials by using trimethyl silicon, then performing butt joint on the raw materials together with 1,2,3,5-tetra-O-acetyl-beta-D-ribose under the catalysis of Lewis acid trimethylsilyl triflate to obtain glycoside, performing alcoholysis to remove protective groups, and performing recrystallization to obtain the azacitidine. According to the synthetic method disclosed by the invention, the 5-azacytosine and the tetraacetylribose are used as the raw materials, and in the reaction of obtaining the glycoside through butt joint, the Lewis acid trimethylsilyl triflate (TMSOTf) replaces tin tetrachloride, so that the problems that a crude metallic tin is overproof and after treatment causes emulsification easily and is inconvenient are avoided, process raw materials of the synthetic method are easy to obtain, and the synthetic method is simple in operation, high in yield, less in step, environment-friendly, and suitable for industrial production.
Owner:TIANJIN JIUHAI MEDICAL TECH
Process for making 5-azacytosine nucleosides and their derivatives
ActiveUS8212021B2High boiling pointIncrease polarityEsterified saccharide compoundsOrganic active ingredientsSilylation5-azacytosine
Owner:SCINOPHARM TAIWAN LTD
Acitidine for injection and preparation method thereof
InactiveCN107137359AShort reconstitution timeImprove use complianceOrganic active ingredientsPowder deliveryForeign matterIntramuscular injection
The invention belongs to the technical field of Western medicinal preparations, and particularly provides freeze-dried azacitidine powder for injection and a preparation method thereof. Mainly through control over the unit filling volume and optimization on a freeze-drying process, the freeze-dried powder with smaller particle size is prepared; when the freeze-dried powder is applied to intramuscular injection, a suspension prepared from the freeze-dried powder has the smallest particle size and the maximum settling speed rate and is good in stability, easy to absorb and good in patient compliance; when the freeze-dried powder is applied to intravenous injection, the redissolution time is shortest, visible foreign matters meet requirements, few impurities are contained, and the stability is higher.
Owner:LUNAN PHARMA GROUP CORPORATION
Crystallizing and drying method for preparing high-purity azacitidine
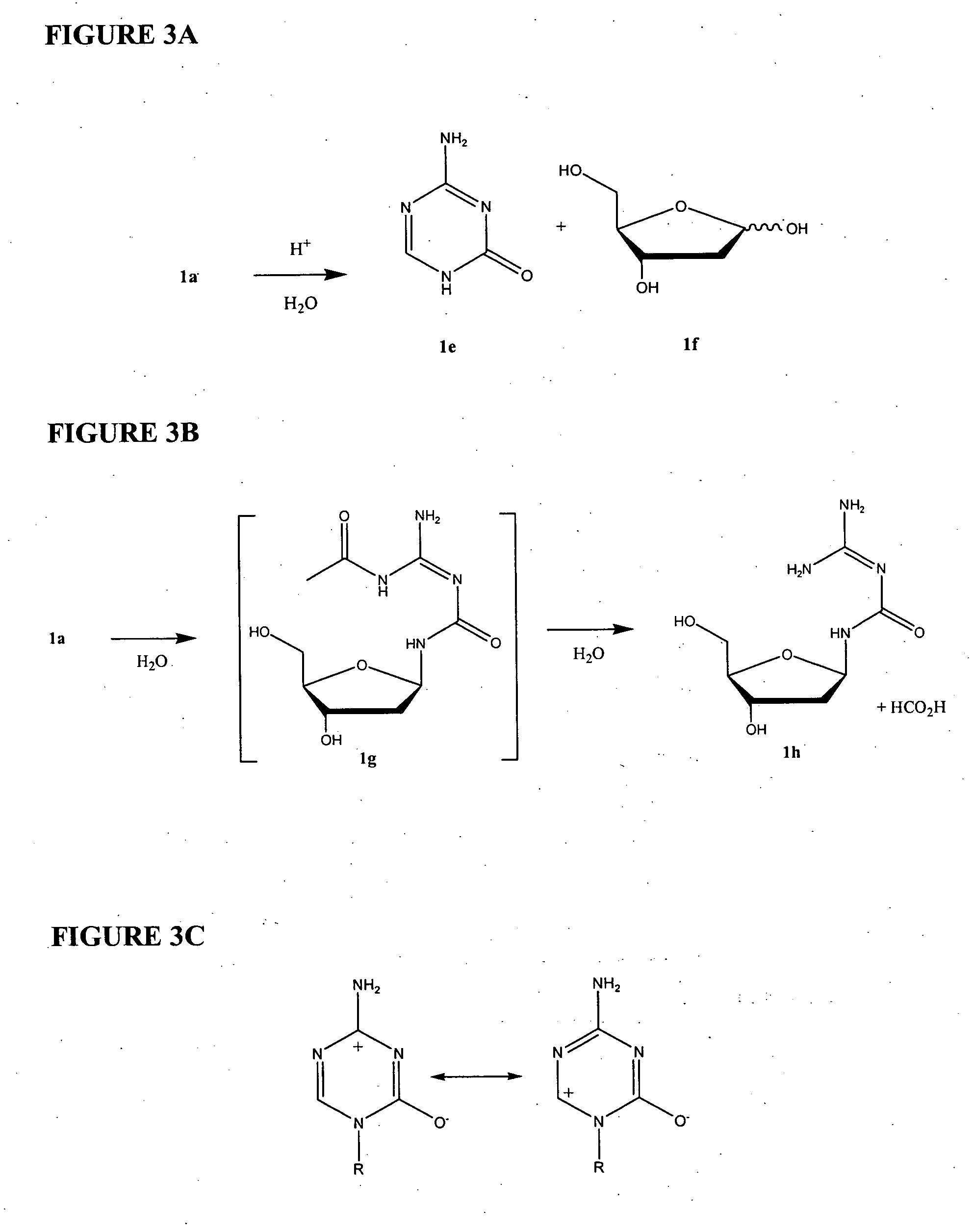
ActiveCN102850418ASuitable for large-scale industrial productionReduce generationSugar derivativesSugar derivatives preparationHydrolysisImpurity
The invention provides a crystallizing and drying method for preparing high-purity azacitidine as myelodysplastic syndrome medicine. The method can reduce the generation of hydrolysis impurity, the obtained product has high purity, and the method is simple, effective, and suitable for large-scale industrial production, and has larger commercial application value.
Owner:杭州容立医药科技有限公司
Stable formulation of azacitidine or salts thereof and their process for preparation
The present invention relates to a stable pharmaceutical composition comprising azacitidine or its pharmaceutically acceptable salts, one or more sugar alcohols and one or more pharmaceutically acceptable excipients, wherein said composition is suitable for parenteral administration. The invention also relates to processes for preparing the preparation of such reconstitutable formulations. The invention also relates to therapeutic methods of use of such formulations and to use of such formulations in the manufacture of medicaments.
Owner:CADILA HEALTHCARE LTD
Preparation method of azacitidine impurity
The invention belongs to the field of medical chemistry, and particularly relates to a preparation method of impurity component cyanurodiamide pentose of azacitidine. The method is characterized in that N2, N4-bi(trimethylsilyl)-6-trimethylsilyl-1,3,5,- triazine-2,4-diamine which is adopted as a raw material has condensation reaction with tetra-o-acetyl-d-ribose under the protection of trimethylsilyl and under the catalysis of aluminium trichloride, boron trifluoride, zinc chloride or titanium tetrachloride at the temperature of -10 to 20 DEG C, and then a protecting group on the sugar is removed to prepare the cyanurodiamide pentose.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Stable azacitidine freeze-drying preparation and preparing method thereof
ActiveCN109646410AFast dissolutionReduce degradationPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLOrganic solvent
The invention discloses a stable azacitidine freeze-drying preparation and a preparing method thereof, belongs to the technical field of pharmaceutical preparations, and particularly provides a stableazacitidine freeze-drying preparation for injection and a preparing method thereof. The freeze-drying preparation comprises azacitidine and mannitol; medicine liquid before free-drying also containsan organic solvent and water for injection. The organic solvent is selected from a mixed solution of teritary butanol, isopropanol and propanediol. By means of the freeze-drying preparation, the dissolution speed of azacitidine is greatly increased, the stability of azacitidine is greatly improved, and the redissolving time of the freeze-drying preparation is shortened.
Owner:JIANGSU HANSOH PHARMA CO LTD
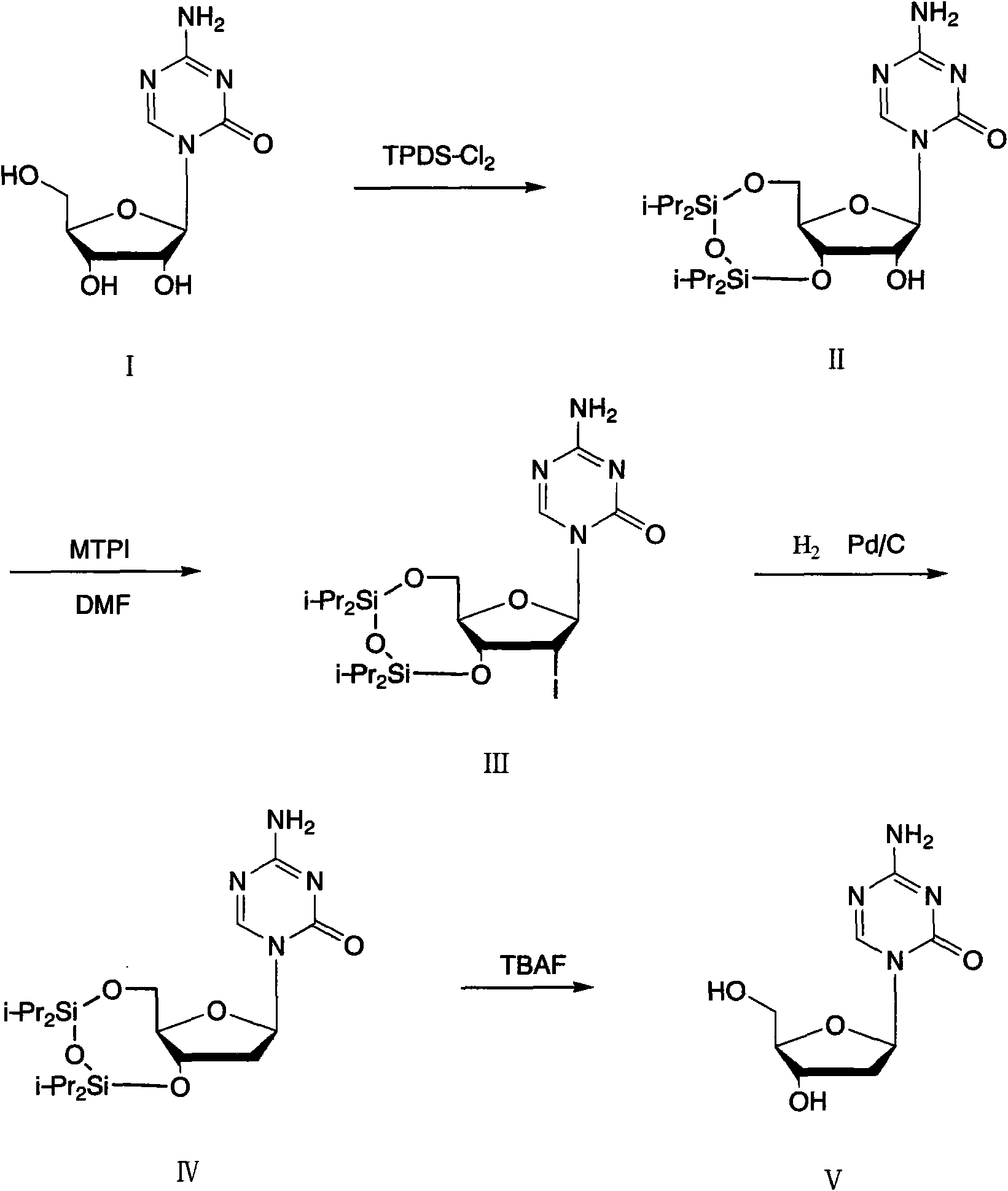
Method for preparing decitabine
ActiveCN102485737AReduce usageEasy to operateSugar derivativesSugar derivatives preparationDisiloxaneSilane compounds
The invention provides a method for preparing decitabine, which takes azacitidine as a raw material, the method comprises the following steps: reacting with 1,3-dichloro-1,1,3,3,-tetraisopropyldisiloxane (TPDS-C12) by taking pyridine as a solvent to prepare silane compounds, then reacting with methyl triphenoxyl phosphonium iodide (MTPI) to obtain 2-iodine substituent, reducing by introducing H2 under Pd / C catalysis to obtain the deoxidant, reacting with tetrabutylammonium fluoride (TBAF) and carrying out deprotection to obtain the decitabine. The product purity can reach 99%, the overall yield is 62%, the technology operation is simple and easy for large scale production realization.
Owner:苏州科耐尔医药科技有限公司
Azacitidine freeze-drying powder needle for injection and method for preparing azacitidine freeze-drying powder needle
InactiveCN108295034AExcellent total impurity levelReduce genotoxic potentialOrganic active ingredientsPowder deliveryOrganic solventFreeze-drying
The invention discloses an azacitidine freeze-drying powder needle for injection, the azacitidine freeze-drying powder needle comprises the raw materials, by weight, of 2-8 parts of azacitidine, 2-8 parts of mannitol and 150-400 parts of organic solvent. The invention further provides a method for preparing the azacitidine freeze-drying powder needle for injection. According to the azacitidine freeze-drying powder needle for injection and the method for preparing the azacitidine freeze-drying powder needle, by changing the technical key points of the product solvent formula, the solution preparation temperature, the charging sequence, the freeze-drying process and the like, the impurity level of the product can be effectively controlled, and the freeze-drying preparation with the stabilitysuperior to that of the foreign original research products can be produced.
Owner:KINDOS PHARM CO LTD +1
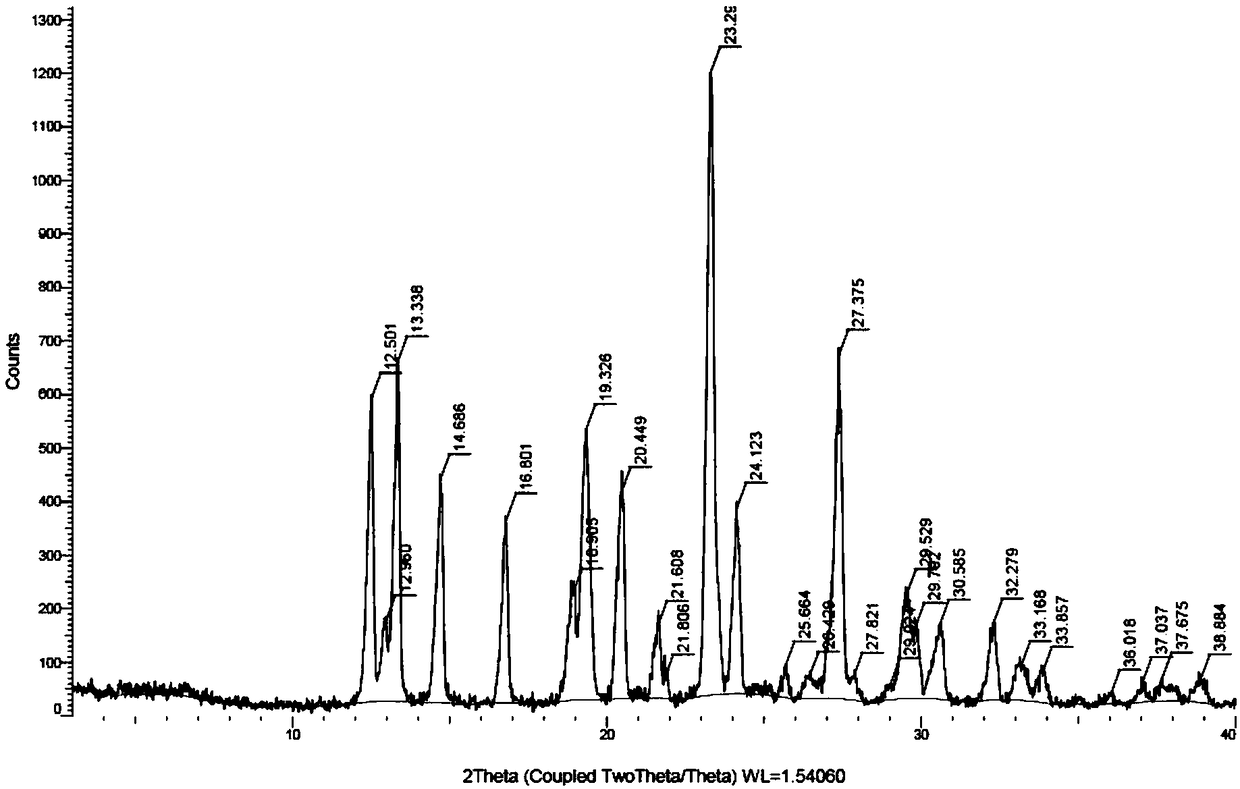
Preparation method for azacitidine crystal form I
ActiveCN108929355AGood effect of removing impuritiesHigh puritySugar derivativesOrganic chemistry methodsAlcoholSolvent
The invention provides a preparation method for azacitidine crystal form I. The preparation method is to put an azacytidine crude product into a mixed solvent including three solvents which are a polar non-protonic solvent, C2-C4 alcohol and water to perform recrystallization, so that the azacitidine crystal form I which has high purity, low impurity content and nearly no solvent residue can be obtained.
Owner:NANJING SHUNXIN PHARM CO LTD OF CHIATAI TIANQING PHARM GRP +2
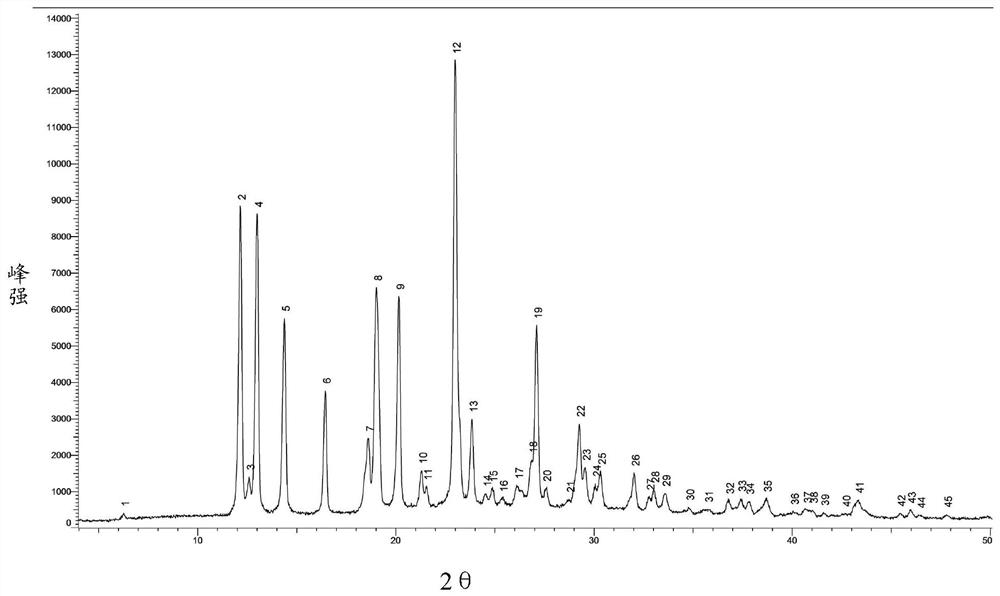
Preparation method of azacitidine crystal form
The invention relates to a preparation method of an azacitidine crystal form I. The preparation method includes: using DMSO as a solvent and a mixed alcohol solvent as an anti-solvent. The crystal form prepared by the method is unitary, high in purity, free of crystal mixing, low in solvent residue and suitable for industrial application and production.
Owner:JIANGSU HANSOH PHARMA CO LTD
Method for preparing azacitidine by high-purity and low-calcination residue
InactiveCN110092807AAdded melting point quality controlReduce dosageSugar derivativesSugar derivatives preparationChemistryAzacitidine
The invention relates to the field of a pharmaceutical synthesis technology, and discloses a method for synthesizing azacitidine. The method improves the quality and product purity of azacitidine, andthe reaction conditions are easy to control and reduce the production cost, and the method is suitable for industrial preparation.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
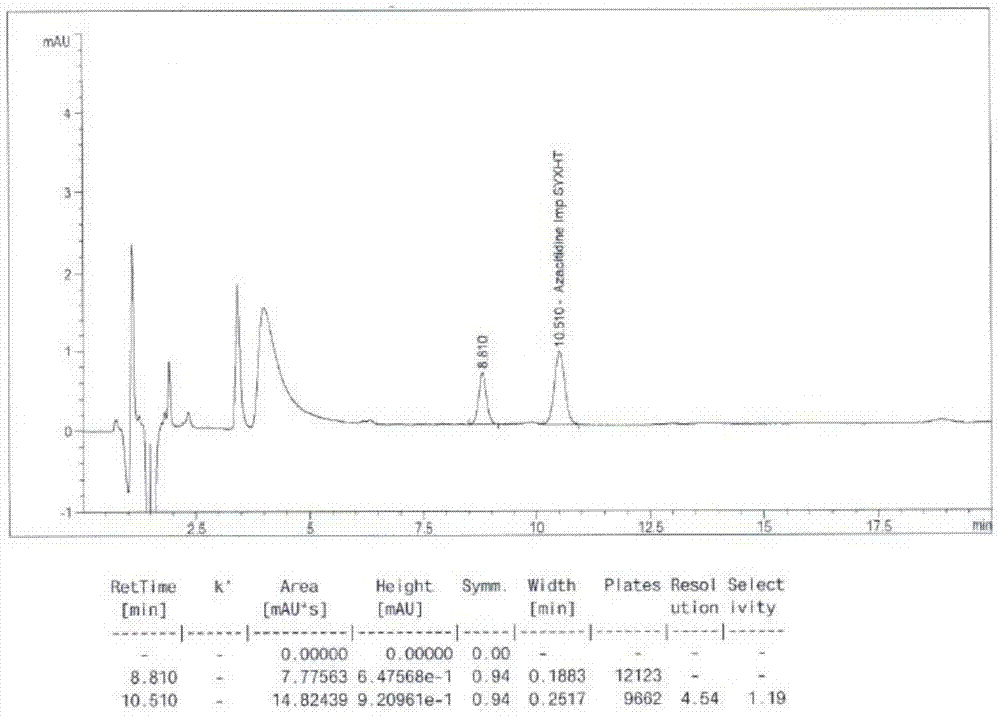
Azacitidine disaccharide impurity and preparation method and application thereof
The invention relates to azacitidine disaccharide impurity and a preparation method and an application thereof. According to the invention, a compound shown as a formula IV is used as a starting material, the compound as a formula III is prepared by catalysis of Lewis acid with tetraacetylribofuranose, and the compound as the formula III is hydrolyzed to prepare the compound as a formula II. The prepared compound as the formula II can be used as a reference for the detection of related substances of azacitidine, and can be used for quality control application of azacitidine and its related preparations.
Owner:WUHU SIMCERE ZHONGREN PHARM +2
Refining method of azacitidine with high purity and low residual solvent content
ActiveCN112300222AEfficient removalResidue reductionSugar derivativesSugar derivatives preparationPhysical chemistryAzacitidine
The invention relates to the field of medicines and chemistry, and provides an azacitidine refining method. The method comprises the steps of 1, adding an azacitidine crude product to a good solvent for dissolving; 2, adding a poor solvent into a solution obtained in the step 1, and performing filtration to obtain an azacitidine primary refined product; and 3, adding the azacitidine primary refined product obtained in the step 2 into the good solvent for dissolving, adding the poor solvent, and performing filtration, pulping and drying to obtain an azacitidine refined product. The method is mild in refining process condition, simple to operate, suitable for industrial amplification and high in product purity; and the content of the residual solvent, especially the residual solvent DMSO with high boiling point, in the product is low.
Owner:北京益佰医药研究有限公司 +1
Azacitidine lyophilized powder for injection and preparation method thereof
ActiveCN109820827AExcellent levelReduce spawn ratePowder deliveryOrganic active ingredientsMANNITOL/SORBITOLFreeze-drying
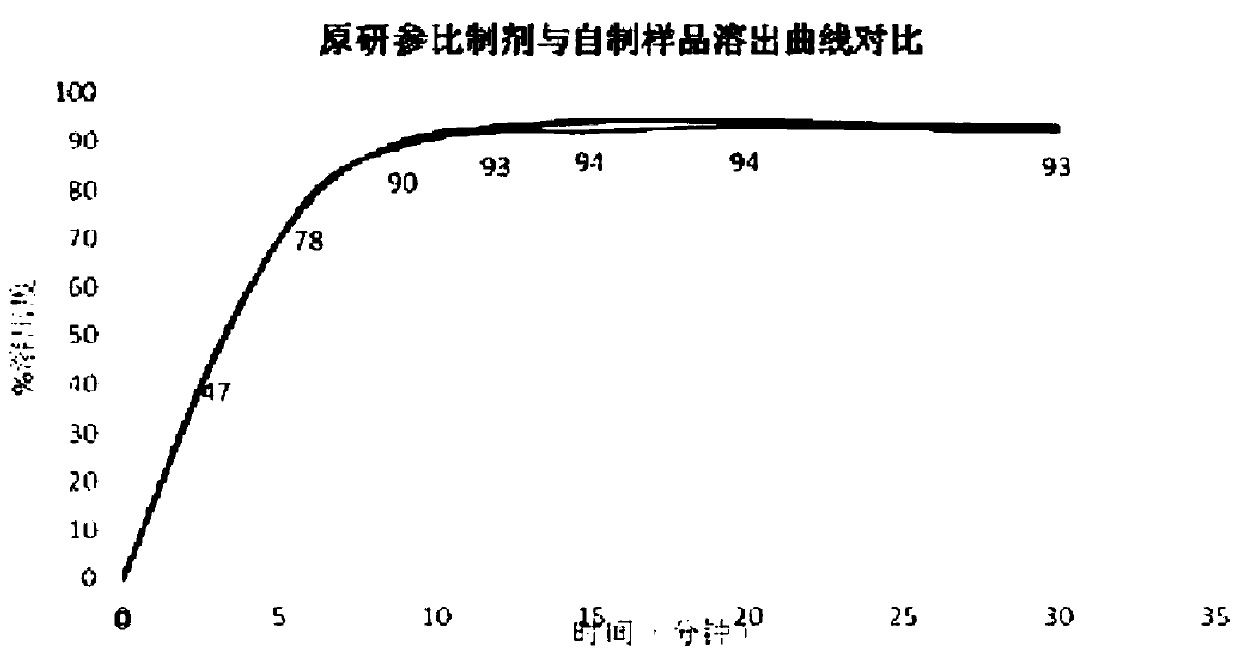
The invention provides azacitidine lyophilized powder for injection. The azacitidine lyophilized powder is prepared from, by weight, 2-8 parts of azacitidine, 2-8 parts of mannitol, 100-300 parts of acetonitrile and 684-896 parts of water. The invention further provides a preparation method of the azacitidine lyophilized powder for injection. The preparation method comprises the steps that part ofthe water for injection is added into a liquid preparation tank; 100-300 parts by weight of acetonitrile is added, and stirring is conducted until the mixture is clear; 2-8 parts by weight of mannitol is added, stirring is conducted until the mixture is completely dissolved, and the temperature is lowered to be 0-4 DEG C; 2-8 parts by weight of a pre-micronized raw azacitidine material is weighed, washed with part of the remaining water for injection and then added into the liquid preparation tank, intensive stirring is conducted until the mixture is dissolved, and the water for injection isadded to reach the constant volume of 1000 parts; after sterile filtration, filling and freeze-drying, the preparation product is obtained. The particle size of the product and the total impurity level of the product can be effectively controlled, the stripping curve and other bioequivalence indexes of the product are equivalent to those of an original research product, and the total impurity level is lower than that of the original research product.
Owner:KINDOS PHARM CO LTD +1
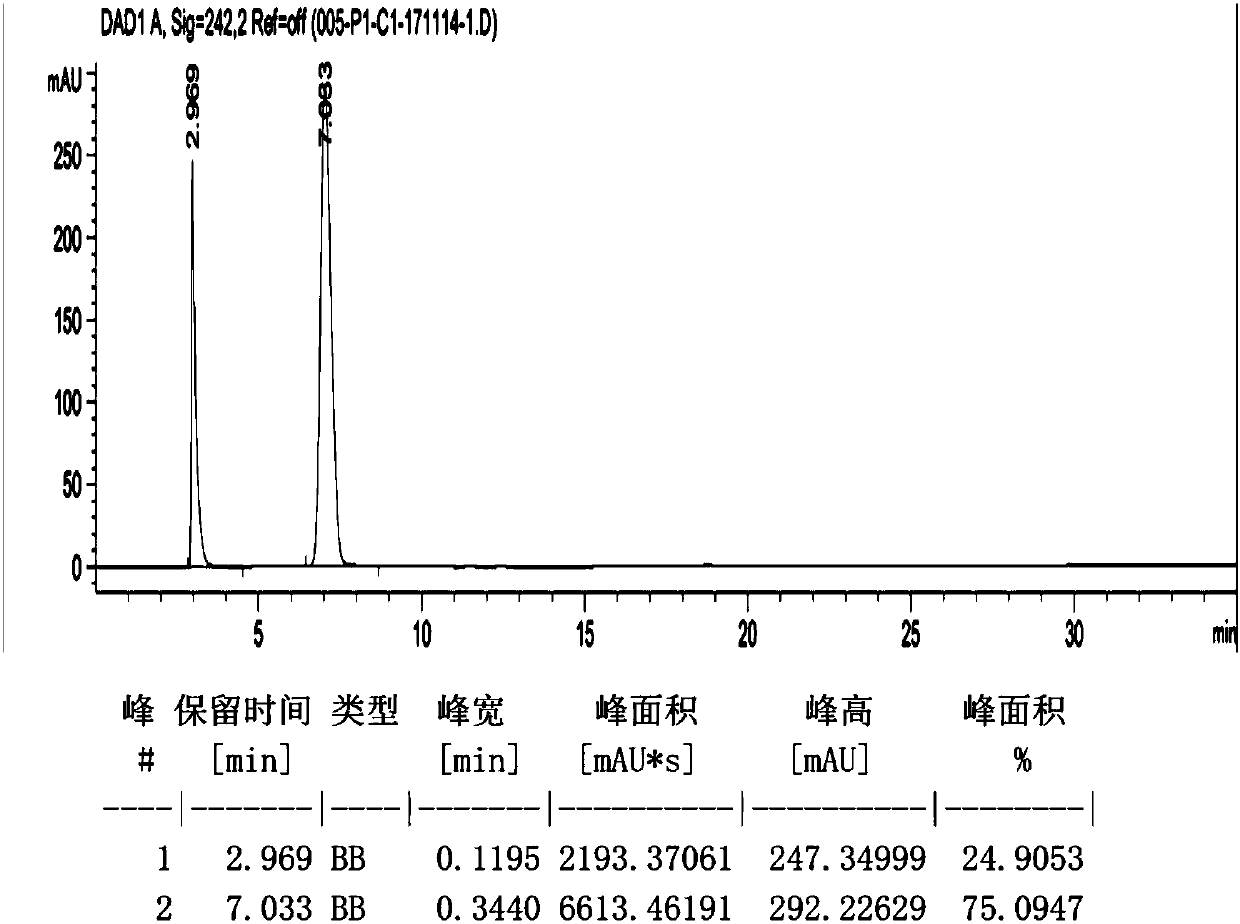
Detection method for tetraacetylribose in azacitidine raw material
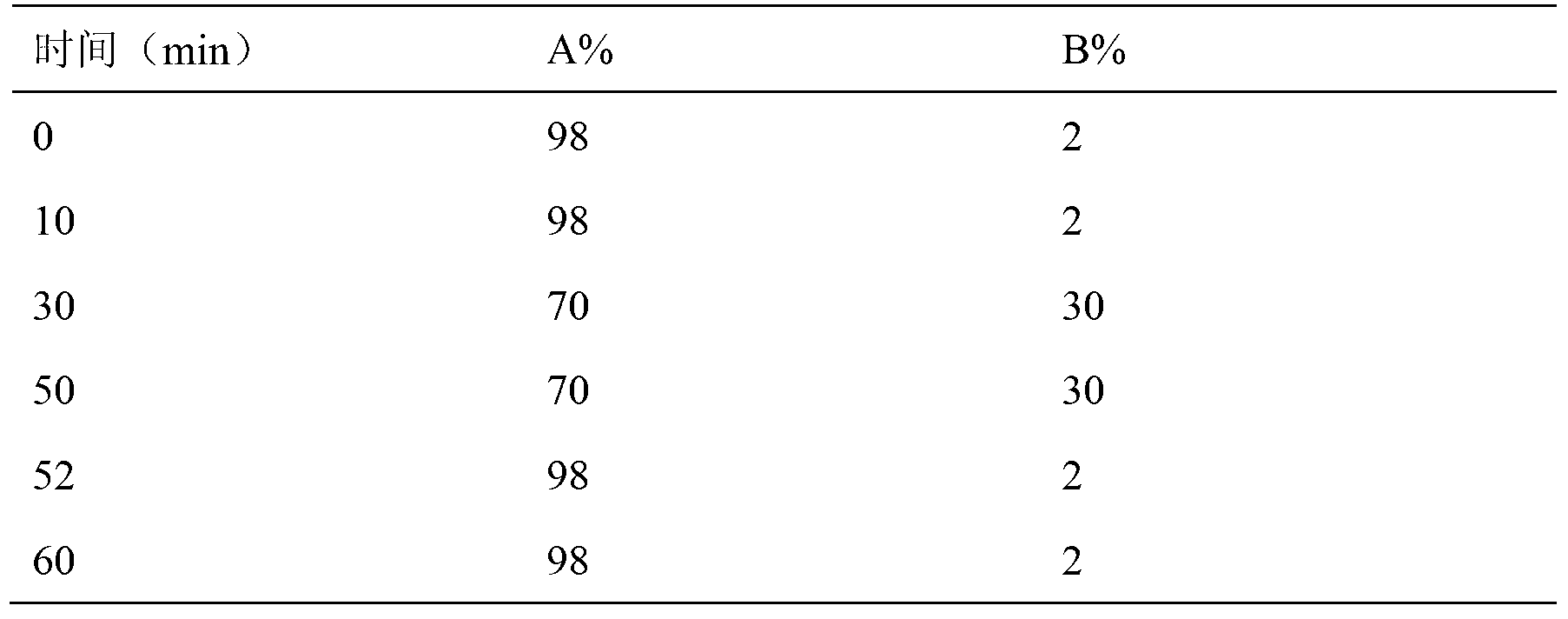
InactiveCN104297361AAccurate detectionSimple methodComponent separationWater methanolIsocratic elution
The invention discloses an impurity detection method, in particular to a detection method for tetraacetylribose in azacitidine. The method adopts high performance liquid chromatography, a octadecyl silane bonded silica gel chromatographic column is selected, and water-methanol is employed as the mobile phase to conduct isocratic elution, the flow rate is 0.8-1.2mL / min, the column temperature is 25-35DEG C, and the detection wavelength of the sample is 205nm-215nm. The mobile phase is employed to perform isocratic elution, wherein the volume percentage of methanol is 30-45%. The detection method has the advantages of high sensitivity, high accuracy, good impurity peak shape symmetry, and high column efficiency, etc.
Owner:SICHUAN HUIYU PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com