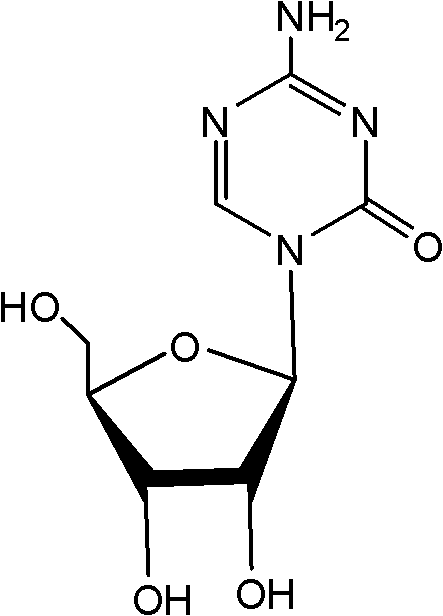
Crystallizing and drying method for preparing high-purity azacitidine
A technology for azacitidine and crude azacitidine, which is applied in the field of crystallization and drying for the preparation of high-purity azacitidine, which can solve the problems of hydrolysis impurities, azacitidine being hydrolyzed in large quantities, and yield reduction, etc. Reduce the production of hydrolyzed impurities, great commercial application value, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of Crude Azacitidine
[0021]
[0022] In a 10-liter three-neck flask, add 6 liters of anhydrous methanol, add 800 grams of triacetylazacitidine, control the internal temperature at 20-30 ° C, add 20 grams of sodium methoxide under stirring, and stir for 16 hours. Cloudy, filtered, the filter cake was washed with 1 liter of methanol, vacuum filtered and dried, and the solid was dried under vacuum with phosphorus pentoxide to constant weight to obtain 250 grams of azacitidine crude product (HPLC normalization method 89%)
[0023] Triacetylazacitidine: {4-amino-1-(2',3',5'-tri-O-acetyl-β-D-ribofuranosyl)-1,3,5-triazine-2 (1H)-ketone}
Embodiment 2
[0025] Crystallization and drying method for preparing high-purity azacitidine
[0026] 83 grams of the crude product obtained in Example 1 was dissolved in 1.45 liters of water preheated to 90°C, 1 gram of activated carbon was added, stirred for 2 minutes, filtered while hot, 10.1 liters of methanol preheated to 45°C was added to the filtrate, and the temperature was lowered. to 40°C, add a small amount of seed crystals, slowly cool down to below 10°C, and keep warm at 0-10°C for 13 hours. After vacuum filtration, the filter cake was vacuum-dried to constant weight at room temperature to obtain about 25 g of dry product (HPLC normalization method 99%).
[0027] The obtained 25 grams of crude product was refluxed with 20 liters of methanol to dissolve, naturally cooled to room temperature, left to stand for crystallization for 3 days, filtered, and the filter cake was vacuum-dried at room temperature to obtain 22 grams of white needle-shaped crystalline powder. (HPLC normaliz...
Embodiment 3
[0033] Crystallization and drying method for preparing high-purity azacitidine
[0034] 83 grams of the crude product obtained in Example 1 was dissolved in 692 milliliters of water preheated to 60 ° C, 1 gram of activated carbon was added, stirred for 2 minutes, filtered while hot, 3458 milliliters of methanol preheated to 65 ° C was added to the filtrate, and the temperature was lowered to 40°C, add a small amount of seed crystals, slowly cool down to below 10°C, and keep warm at 0-10°C for 13 hours. After vacuum filtration, the filter cake was vacuum-dried to constant weight at room temperature to obtain about 50 g of dry product (HPLC normalization method 93%).
[0035] 50 g of the obtained crude product was dissolved in 692 ml of water preheated to 75 °C, 1 g of activated carbon was added, stirred for 2 minutes, filtered while hot, 3458 ml of methanol preheated to 60 °C was added to the filtrate, and the temperature was lowered to 40 °C , add a small amount of seed cryst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com