Azacitidine lyophilized powder for injection and preparation method thereof
A technology of azacitidine and freeze-dried powder injection, which is applied in the field of medicine, can solve the problems of no significant improvement in impurity levels, long dissolution time, and limited inhibitory effect, so as to reduce the residual solvent level of acetonitrile and avoid genotoxicity Risk, the effect of shortening the process time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
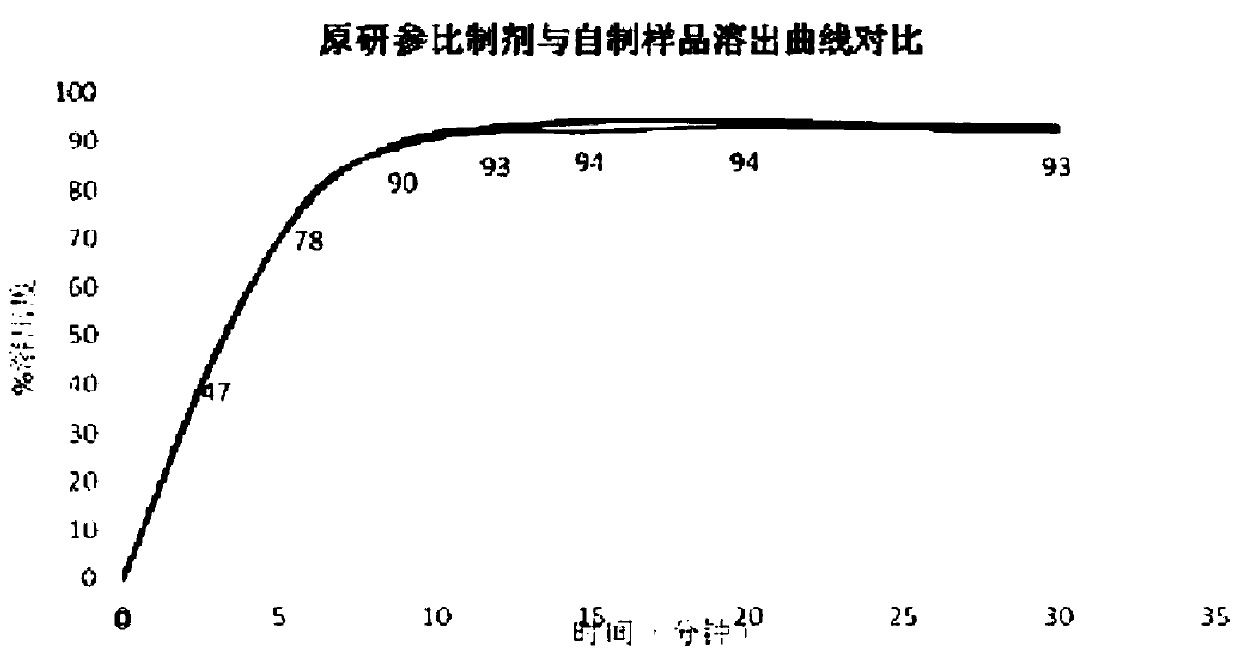
Image
Examples
Embodiment 1
[0031] Azacitidine freeze-dried powder for injection, raw materials include azacitidine, mannitol and acetonitrile, and its weight part formula is: 2g azacitidine, 2g mannitol, 100g acetonitrile and 896g water.
Embodiment 2
[0033] Azacitidine freeze-dried powder for injection, raw materials include azacitidine, mannitol and acetonitrile, and its weight part formula is: 8mg azacitidine, 8mg mannitol, 300mg acetonitrile and 684mg water.
Embodiment 3
[0035]Azacitidine freeze-dried powder for injection, raw materials include azacitidine, mannitol and acetonitrile, and its weight part formula is: 4g azacitidine, 4g mannitol, 140g acetonitrile and 852g water.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com