Patents
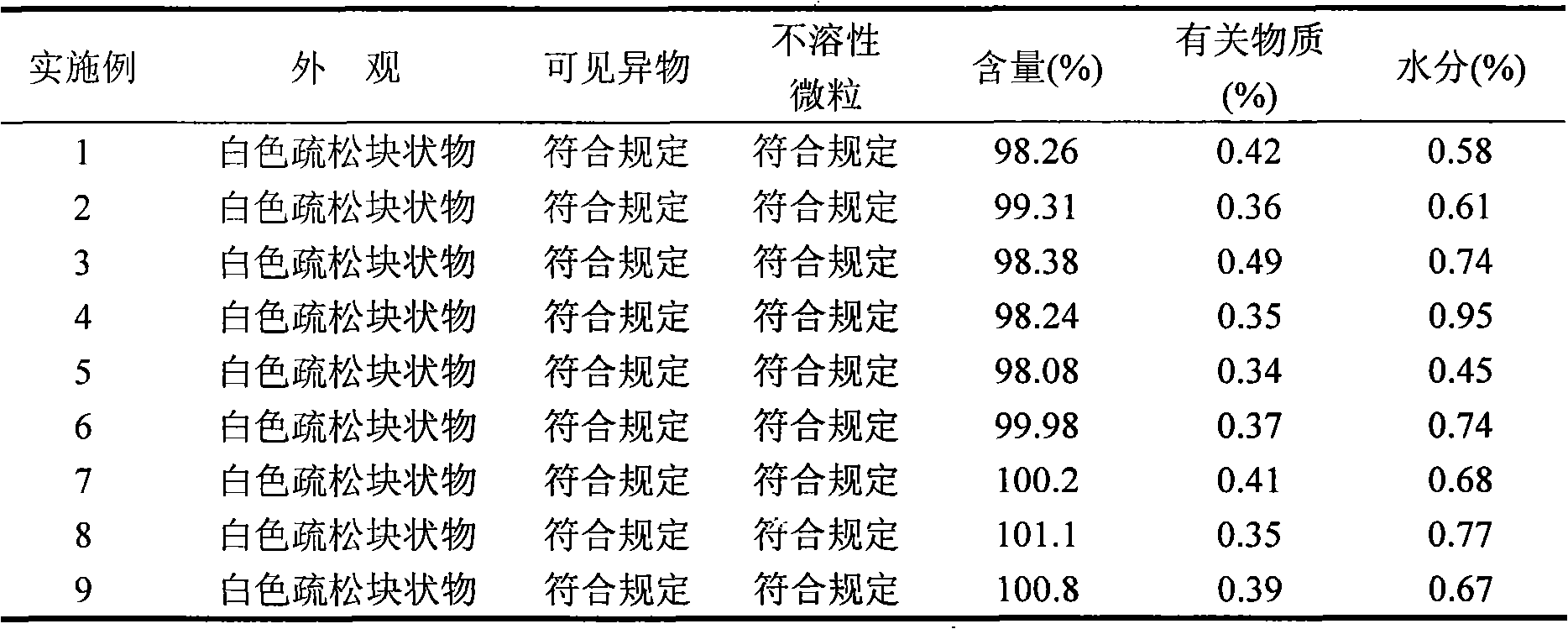
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
275 results about "Myelodysplastic syndromes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A group of disorders resulting from poorly formed or dysfunctional blood cells.
RNA interference mediated inhibition of B-cell CLL/Lymphoma-2 (BCL-2) gene expression using short interfering nucleic acid (siNA)
InactiveUS20050176025A1Improves various propertyImprove the immunityCompounds screening/testingSpecial deliveryAutoimmune conditionAutoimmune disease
This invention relates to compounds, compositions, and methods useful for modulating BCL2 gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of BCL2 gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of BCL2 genes (e.g., BCL2, BCL-XL, BCL2-L1, MCL-1 CED-9, BAG-1, E1B-194 and / or BCL-A1). The small nucleic acid molecules are useful in the treatment of cancer, malignant blood disease, polycytemia vera, idiopathic myelofibrosis, essential thrombocythemia, myelodysplastic syndromes, autoimmune disease, viral infection, and proliferative diseases and conditions
Owner:SIRNA THERAPEUTICS INC
Methods of using JNK or MKK inhibitors to modulate cell differentiation and to treat myeloproliferative disorders and myelodysplastic syndromes
InactiveUS20040028660A1Increase productionPromote recoveryBiocideGenetic material ingredientsCord blood stem cellMyeloproliferative Disorders
The present invention provides methods of modulating mammalian, particularly human, stem cell and progenitor cell differentiation to regulate and control the differentiation and maturation of these cells along specific cell and tissue lineages. The methods of the invention relate to the use of certain small organic molecules to modulate the differentiation of stem cell populations along specific cell and tissue lineages, particularly embryonic-like stem cells originating from a postpartum placenta or stem cells isolated form sources such as cord blood. The invention also relates to the treatment or prevention of myelodysplastic syndrome or myeloproliferative syndrome, or symptoms thereof, comprising administration of JNK or MKK inhibitors, alone or in combination, as well as with or without the use of unconditioned cells or cells conditioned in accordance with other aspects of the invention. Finally, the invention relates to the use of such differentiated stem cells in transplantation and other medical treatments.
Owner:ANTHROGENESIS CORP +1
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of myelodysplastic syndromes
InactiveUS20040220144A1Extension of timeBiocidePeptide/protein ingredientsBULK ACTIVE INGREDIENTActive ingredient
Methods of treating, preventing and / or managing myclodysplastic syndromes are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active ingredient, and / or the transplantation of blood or cells. Specific second active ingredients are capable of affecting or blood cell production. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Methods of using 3-(4-amino-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione for the treatment and management of myelodysplastic syndromes
Methods of treating, preventing and / or managing myclodysplastic syndromes are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active ingredient, and / or the transplantation of blood or cells. Specific second active ingredients are capable of affecting or blood cell production. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Isoindoline compounds and methods of making and using the same
The invention encompasses isoindoline compounds, pharmaceutical compositions comprising them, and methods of their use for the treatment, prevention or management of various diseases and disorders. Examples include, but are not limited to, cancer, inflammatory bowel disease and myelodysplastic syndrome.
Owner:AMGEN INC
Methods for diagnosing and evaluating treatment of blood disorders
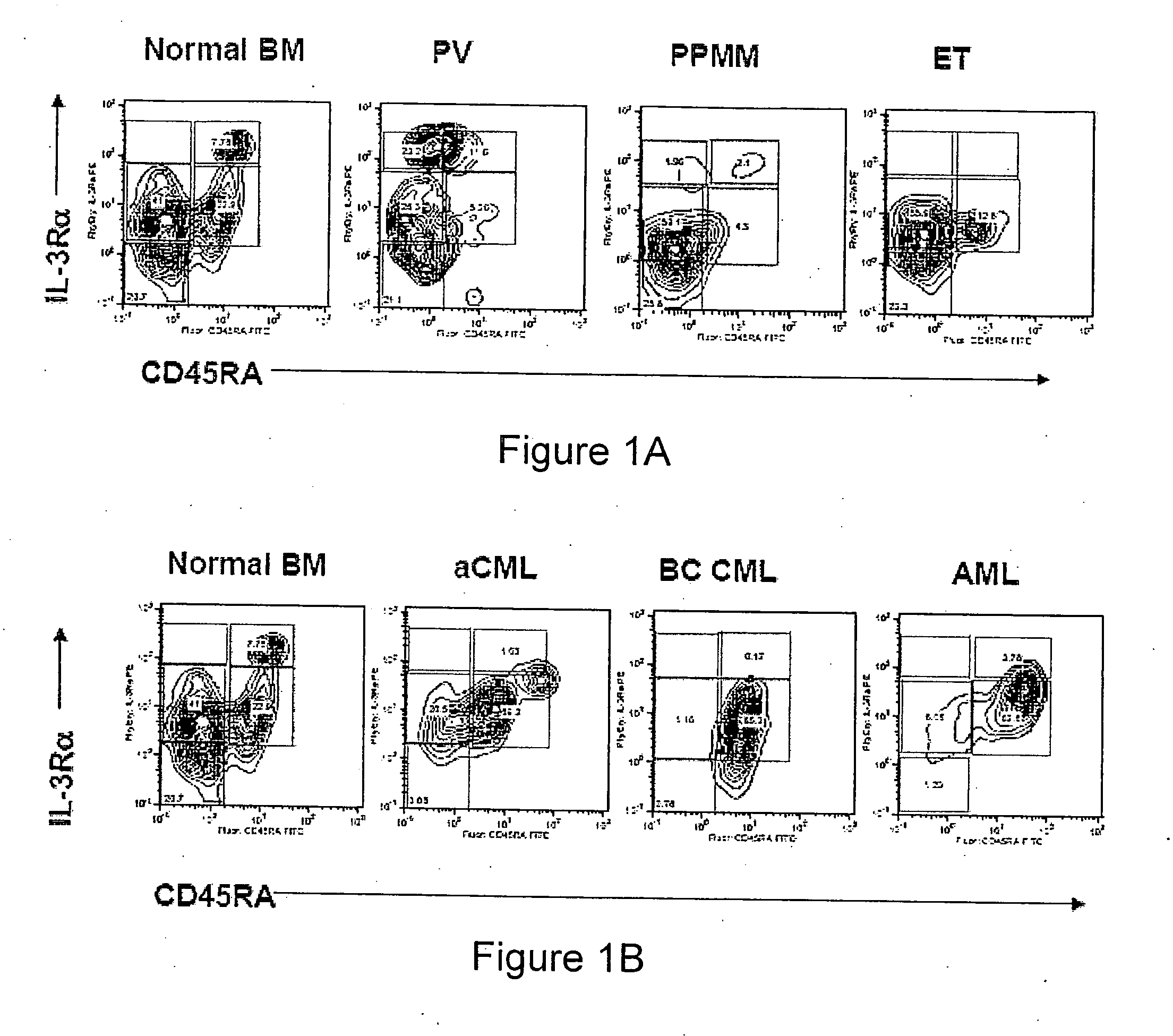
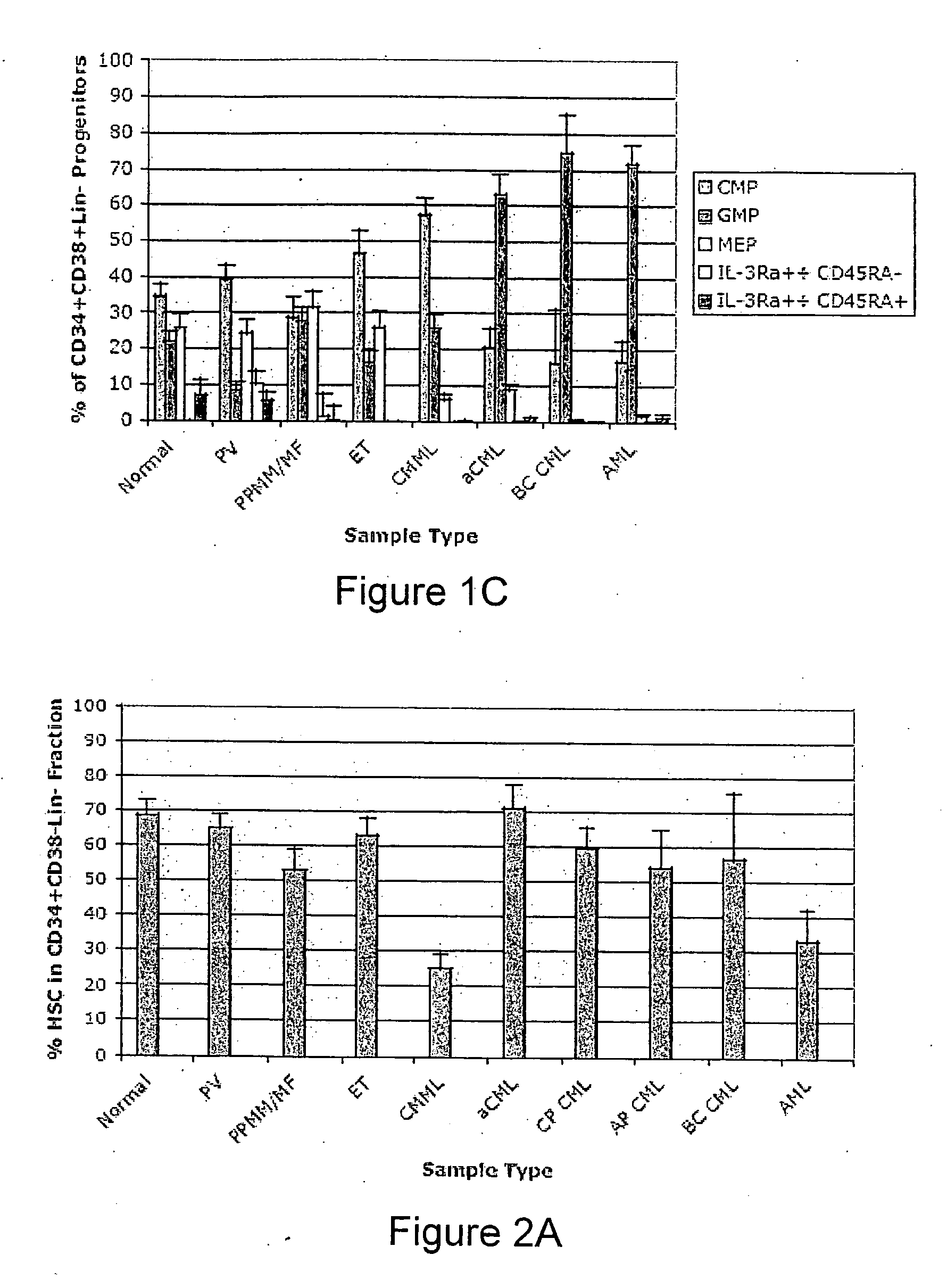
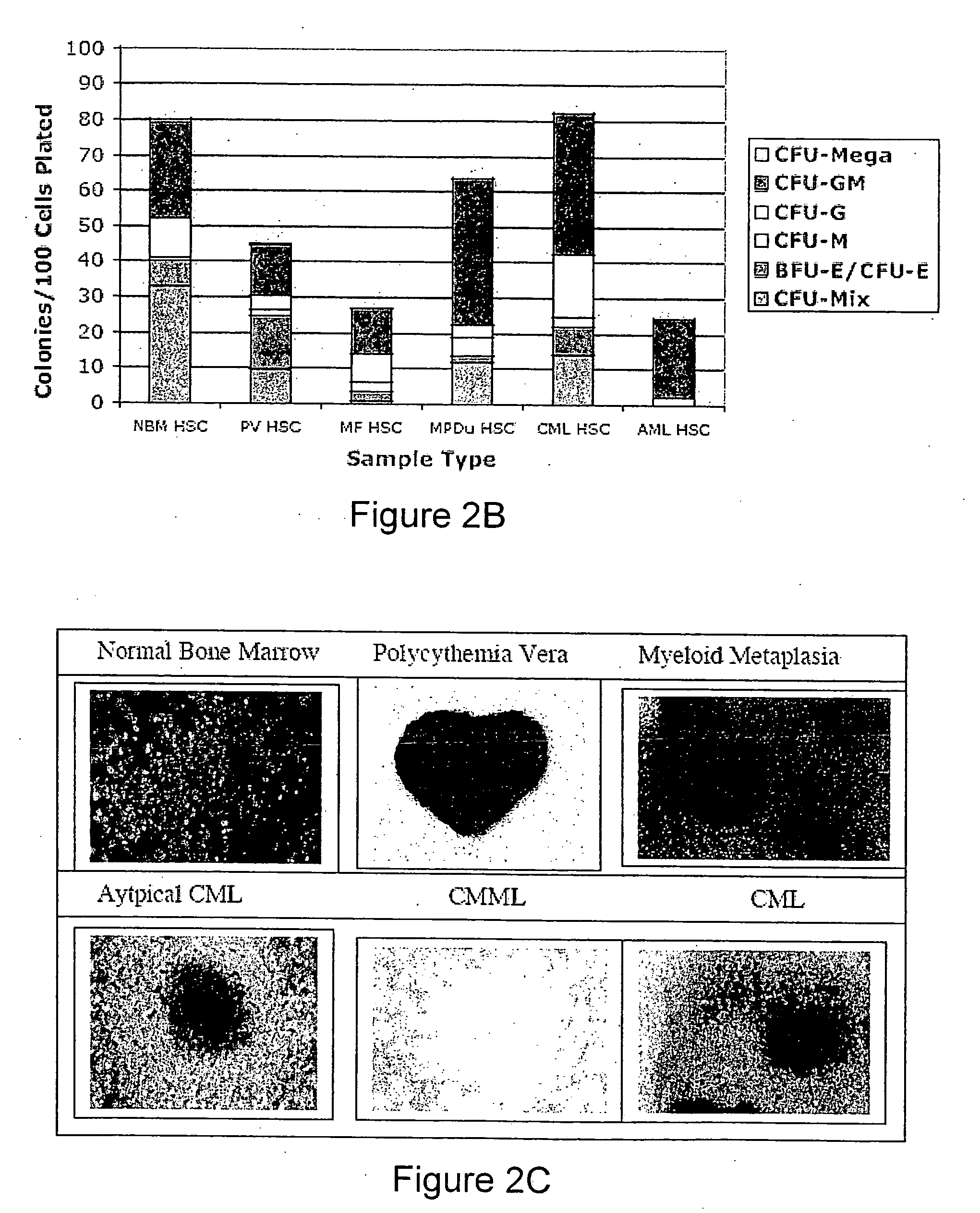
Methods, systems and kits are provided for the clinical staging of blood disorders including myelodysplastic syndrome, myeloproliferative diseases and leukemias by differential analysis of hematologic samples for the distribution of one or more hematopoietic stem or progenitor cell subsets. Additional functional, genetic, gene expression, proteomic or other molecular analyses of the hematopoietic stem and progenitor cells from the patients can also be employed in the staging methods of the invention.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
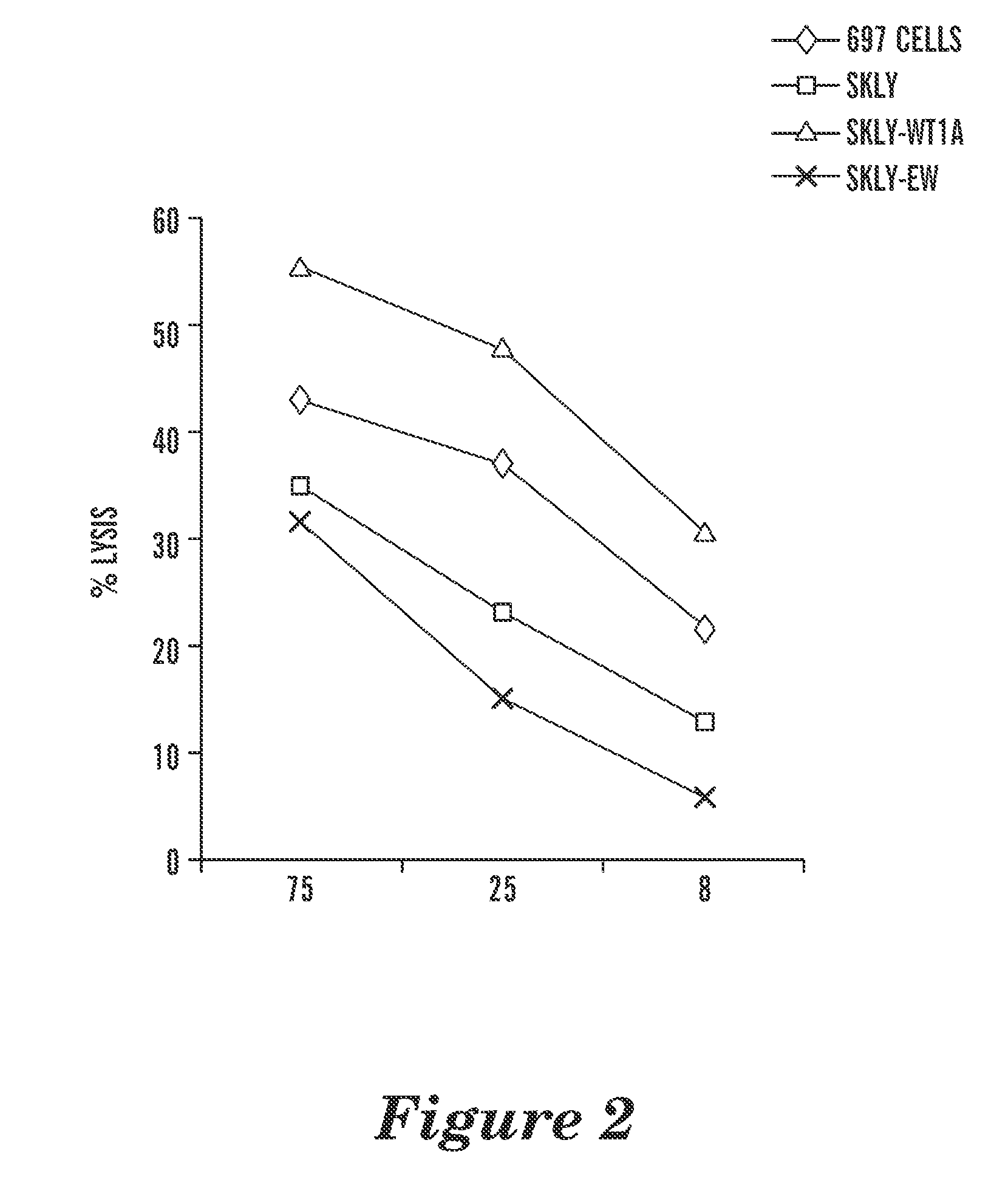
T cell receptor-like antibodies specific for a wti peptide presented by hla-a2
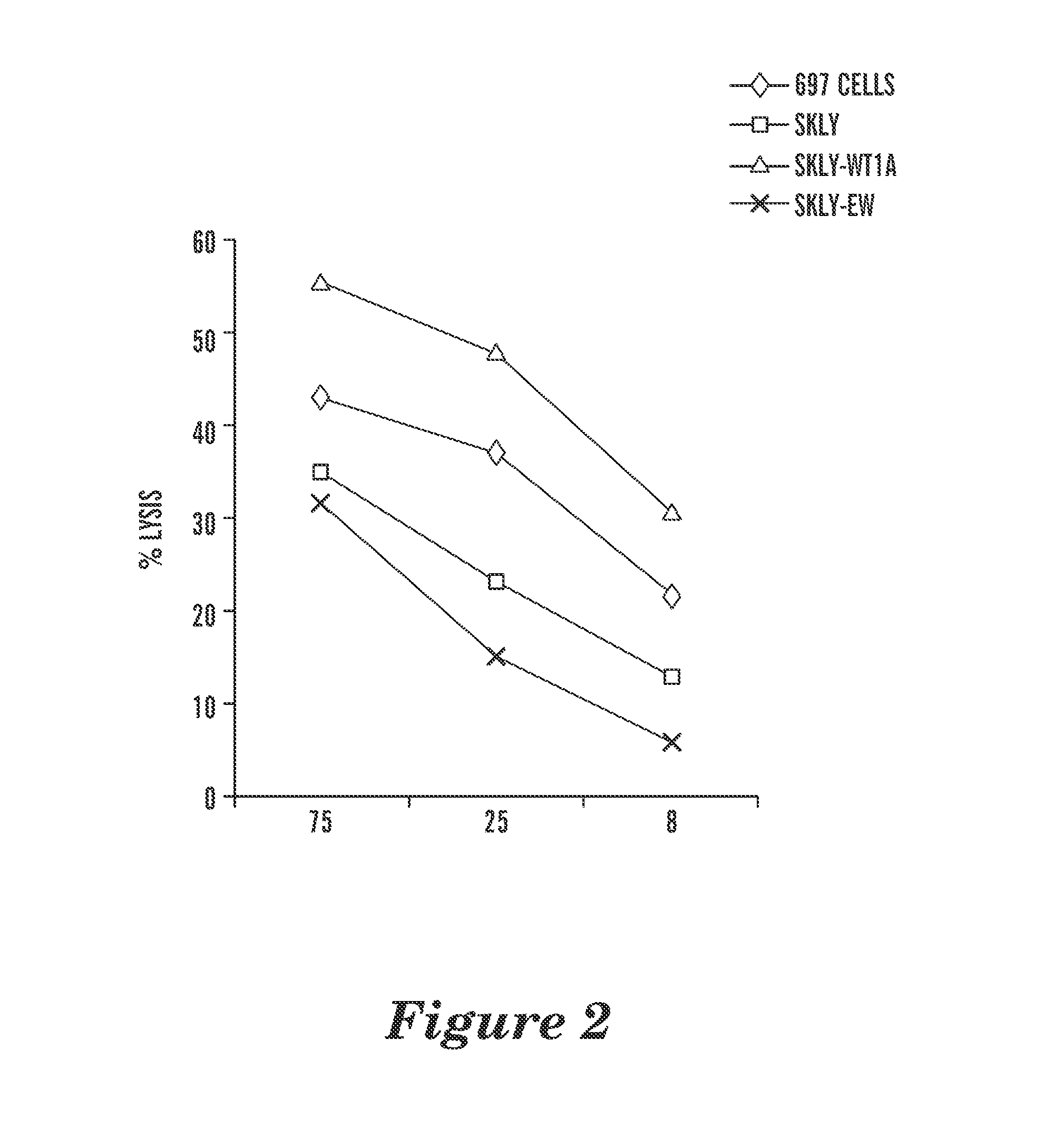
The present invention provides antigen binding proteins that specifically bind to Wilms' tumor protein (WT1), including humanized, chimeric and fully human antibodies against WT1, antibody fragments, chimeric antigen receptors (CARs), fusion proteins, and conjugates thereof. The antigen binding proteins and antibodies bind to HLA-A0201-restricted WT1 peptide. Such antibodies, fragments, fusion proteins and conjugates thereof are useful for the treatment of WT1 associated cancers, including for example, breast cancer, ovarian cancer, prostate cancer, chronic myelocytic leukemia, multiple myeloma, acute lymphoblastic leukemia (ALL), acute myeloid / myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). In more particular embodiments, the anti-WT1 / A antibodies may comprise one or more framework region amino acid substitutions designed to improve protein stability, antibody binding and / or expression levels.
Owner:EUREKA THERAPEUTICS INC +1
Isoindoline compounds and methods of making and using the same
The invention encompasses isoindoline compounds, pharmaceutical compositions comprising them, and methods of their use for the treatment, prevention or management of various diseases and disorders. Examples include, but are not limited to, cancer, inflammatory bowel disease and myelodysplastic syndrome.
Owner:AMGEN INC
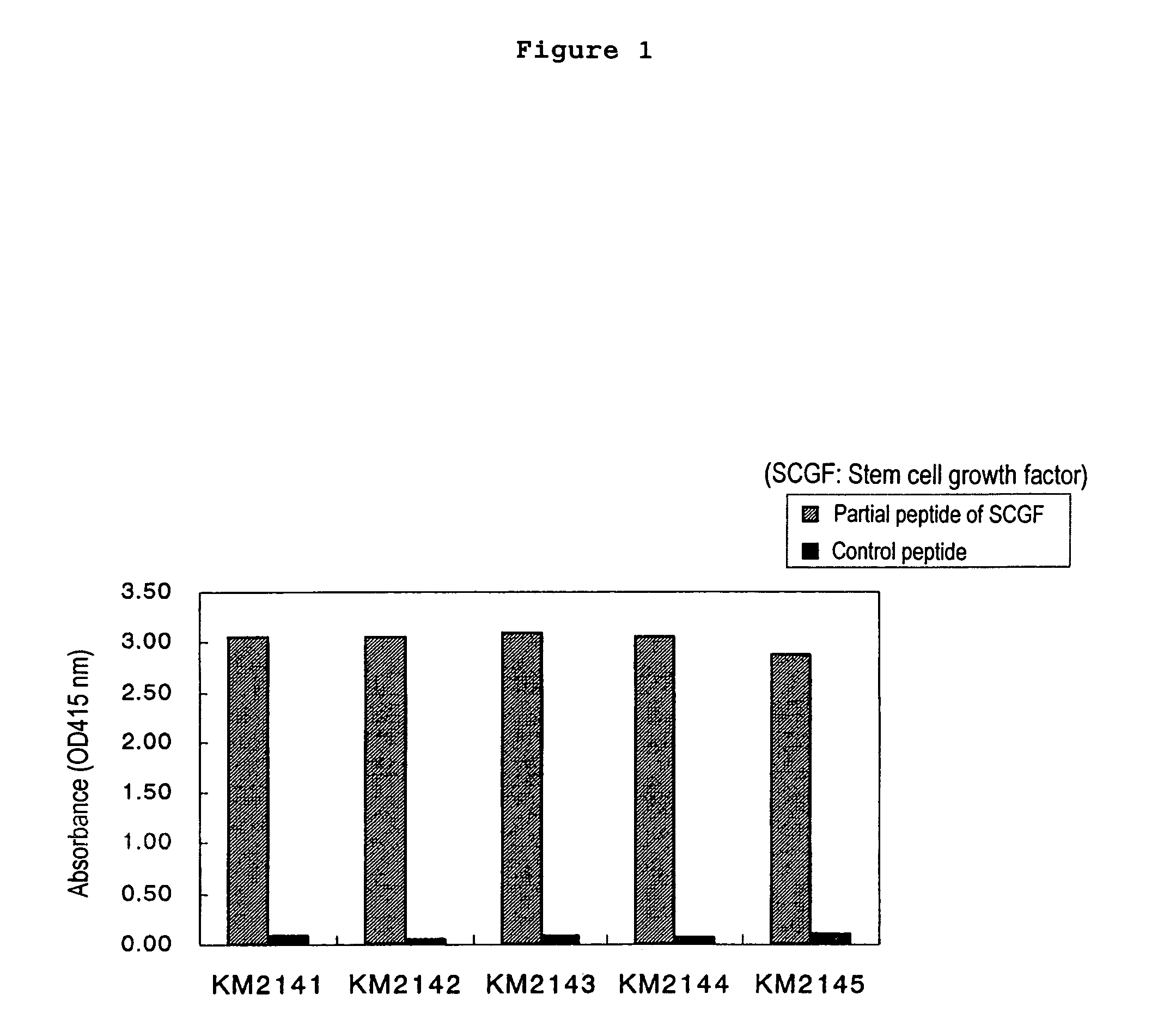
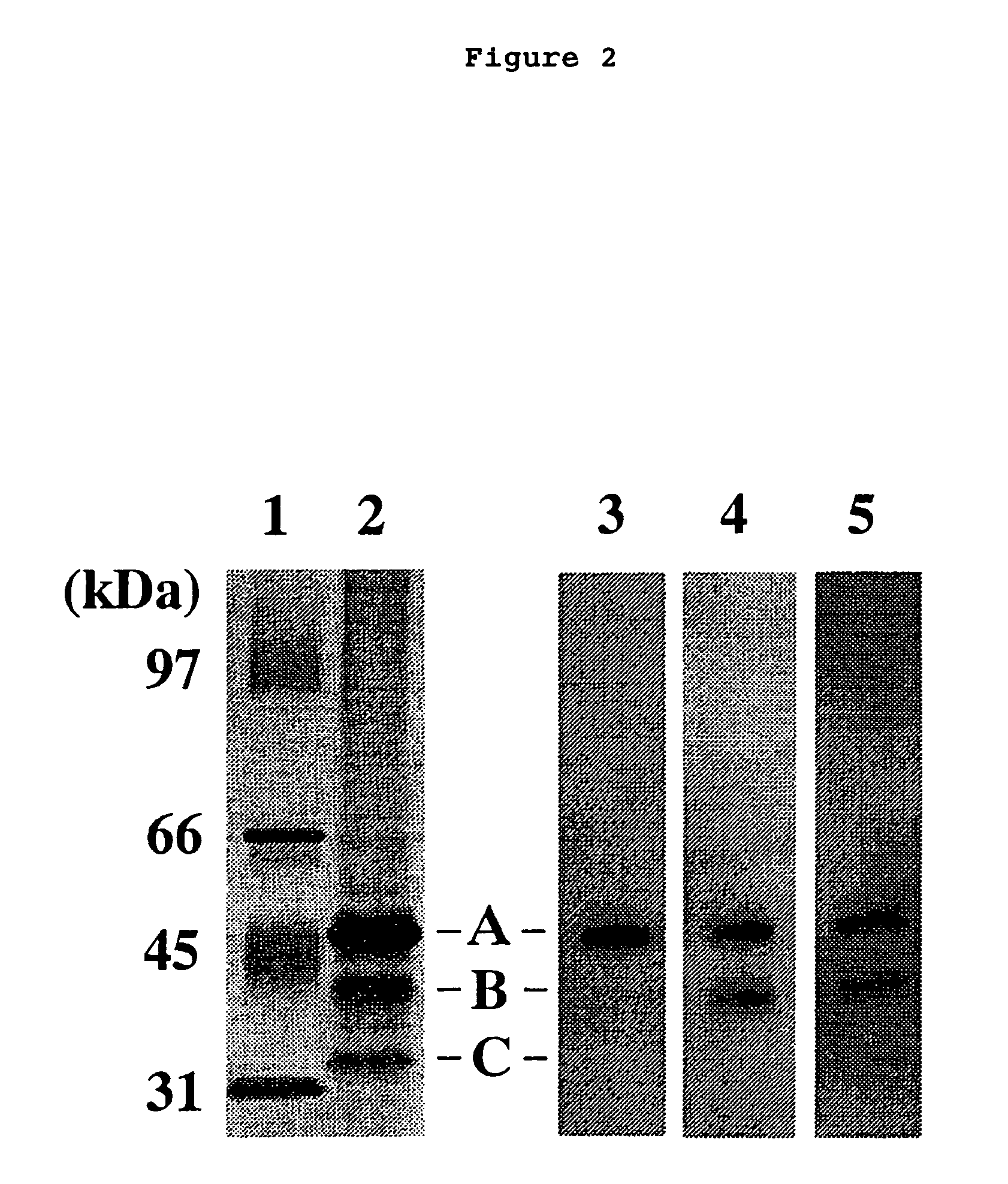
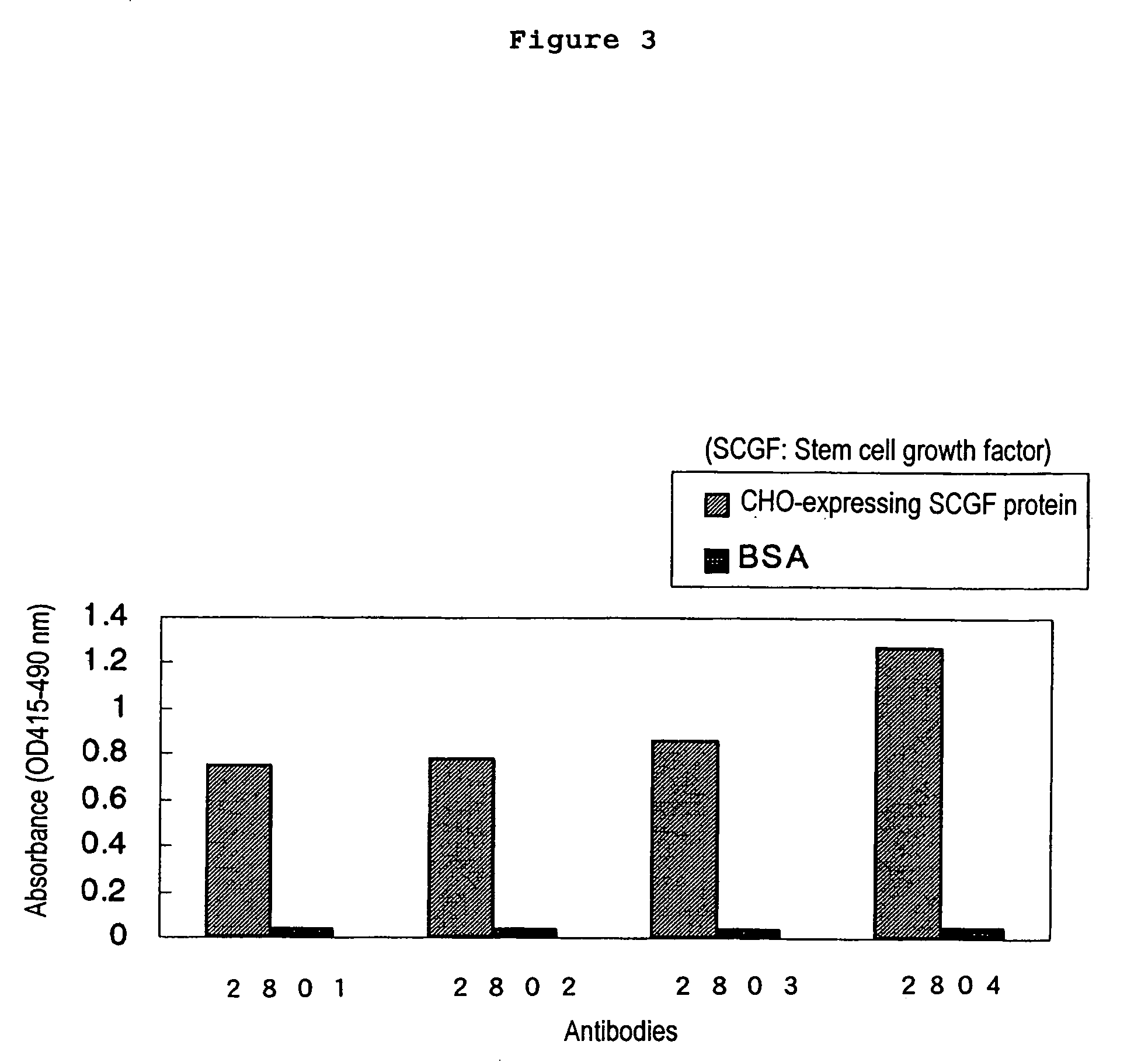
Method of judging leukemia, pre-leukemia or aleukemic malignant blood disease and diagnostic therefor
InactiveUS7479371B2Biological material analysisImmunoglobulins against growth factorsGackstroemiaPreleukemia
The present invention relates to a method for diagnosing leukemia, pre-leukemia or aleukemic malignant blood diseases, a method of discriminating leukemia from pre-leukemia or aleukemic malignant blood diseases, a method of discriminating aplastic anemia from myelodysplastic syndrome, a method of diagnosing delayed engraftment of the hematopoietic stem cells after transplantation of the hematopoietic stem cells, and a method of diagnosing the graft versus host disease, each of said methods comprising quantifying stem cell growth factor (SCGF). The present invention also makes it possible to provide an agent for diagnosing leukemia, pre-leukemia or aleukemic malignant blood diseases and an agent for diagnosing delayed engraftment of the hematopoietic stem cells after transplantation of the hematopoietic stem cells or an agent for diagnosing graft versus host disease (GVHD), each containing as an active ingredient an antibody reacting with stem cell growth factor (SCGF).
Owner:TOKAI UNIV +2
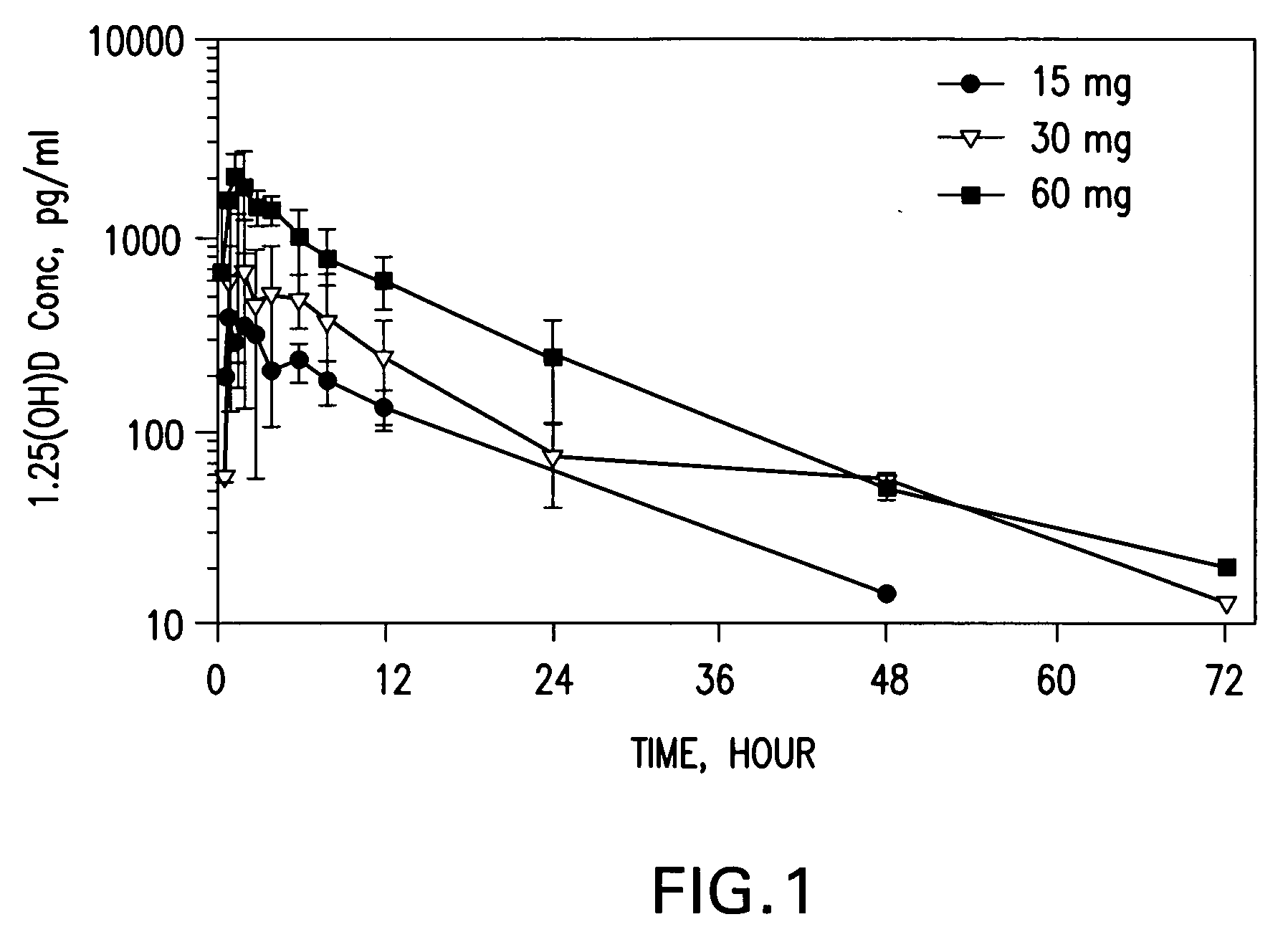
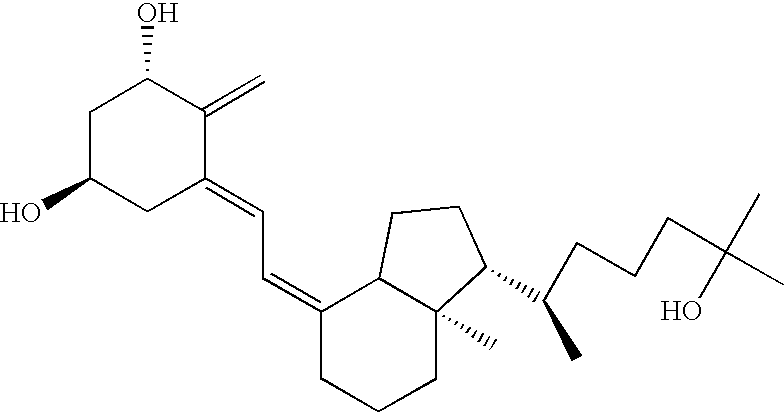
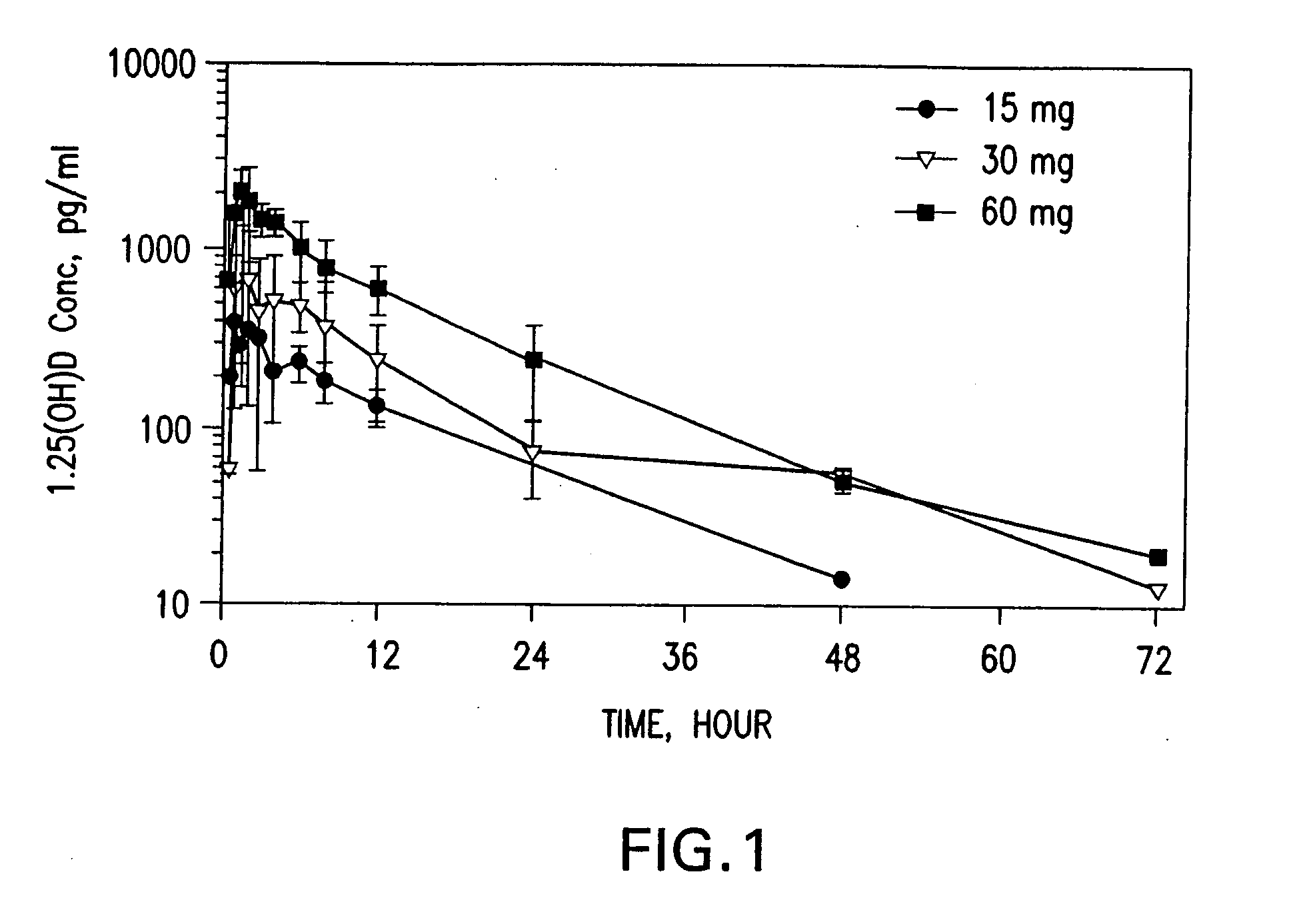
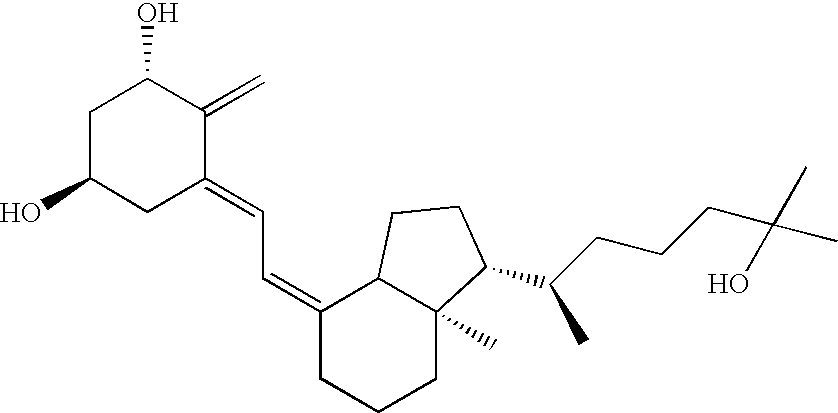
Methods of using vitamin D compounds in the treatment of myelodysplastic syndromes
InactiveUS20050101576A1Minimizing and avoiding effectUseful in treatmentOrganic active ingredientsBiocideActive agentHigh doses
Methods of treating MDS, or ameliorating a symptom thereof, are disclosed. Specific methods encompass the administration of one or more vitamin D compounds, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with one or more additional active agents. Other methods include intermittent administration of a high dose of one or more vitamin D compounds, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with one or more additional active agents. Such intermittent administration allows high doses of the vitamin D compounds to be administered while minimizing or eliminating hypercalcemia.
Owner:NOVACEA INC
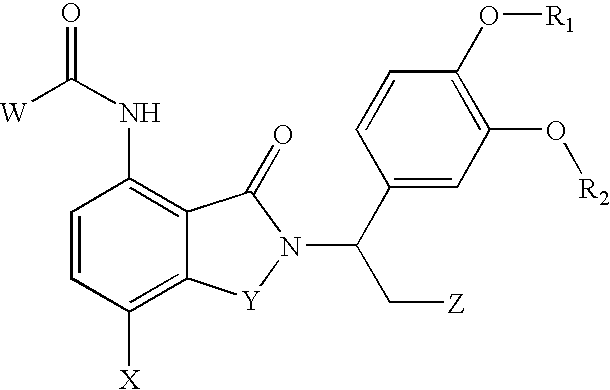
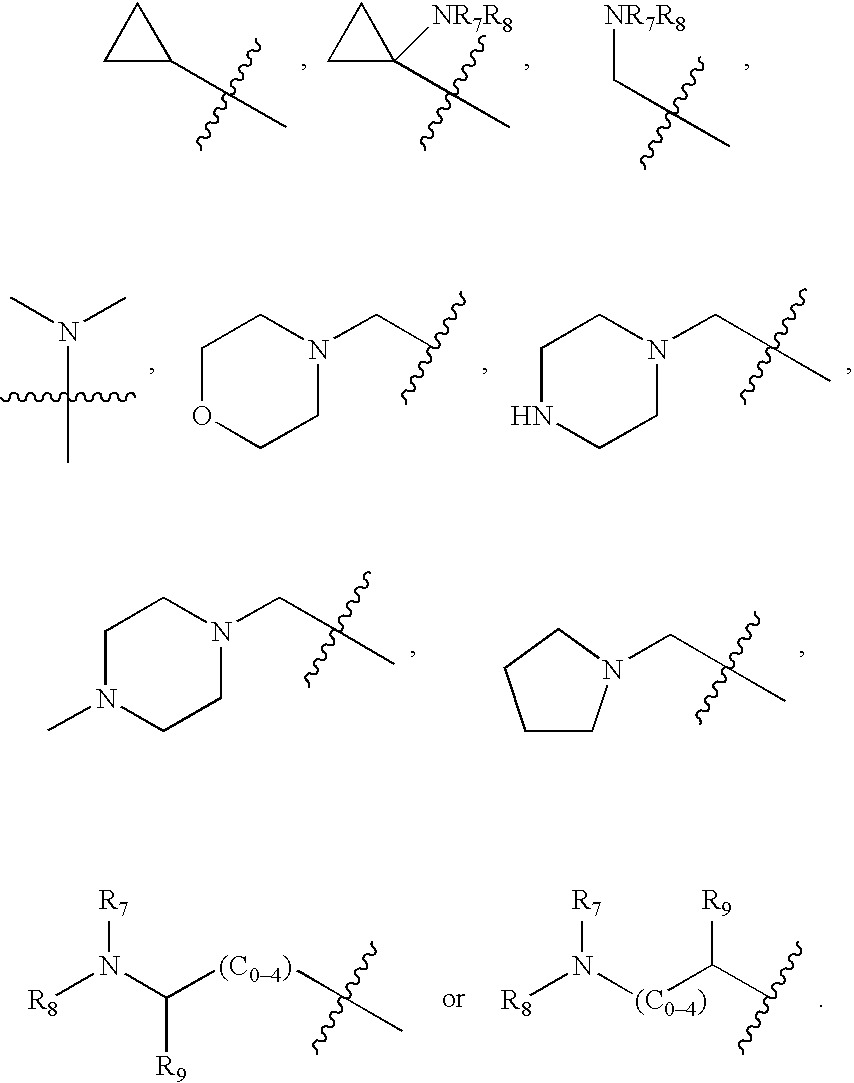
Heterocyclic compounds and their uses
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110δ activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelodysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
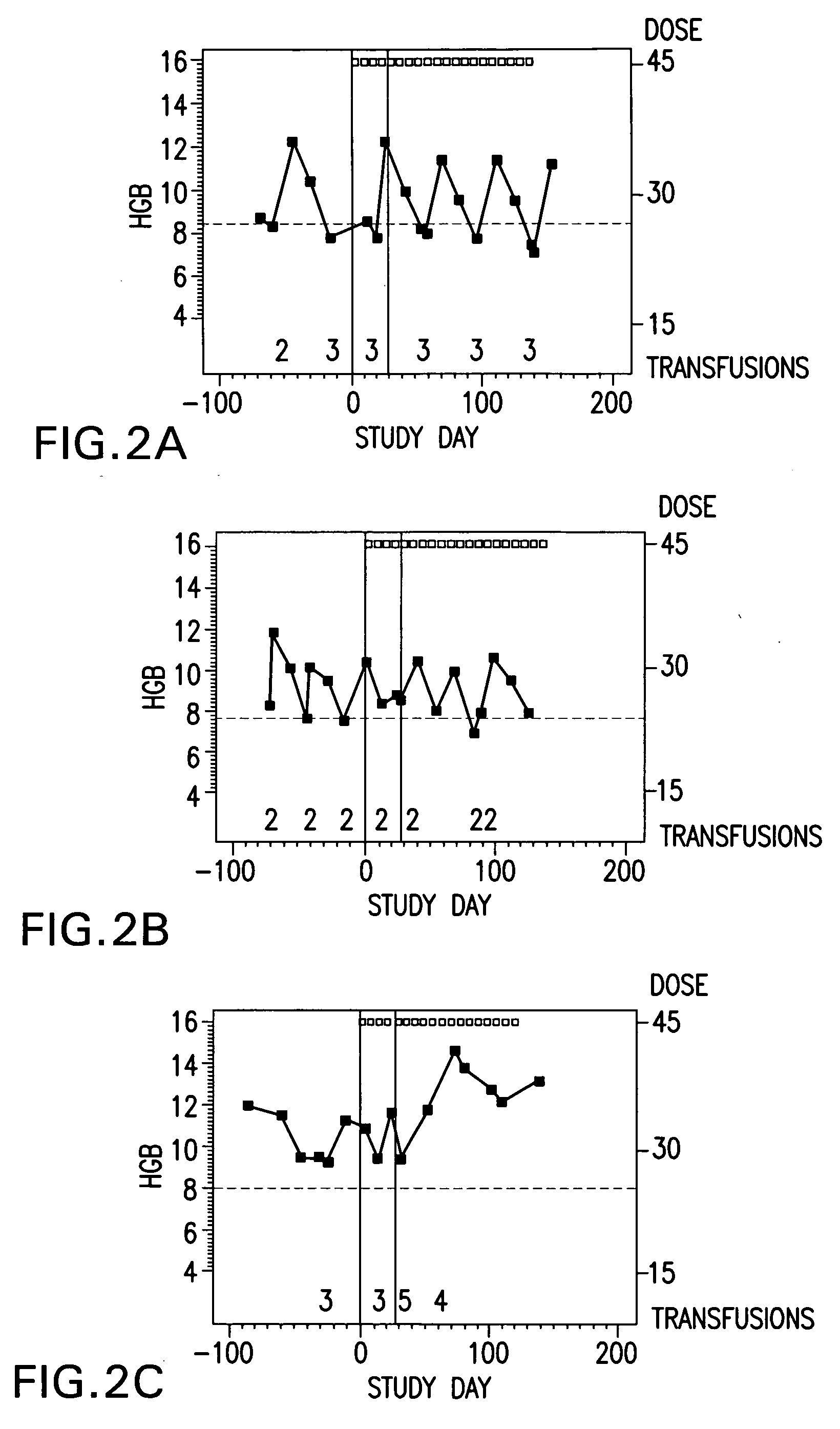
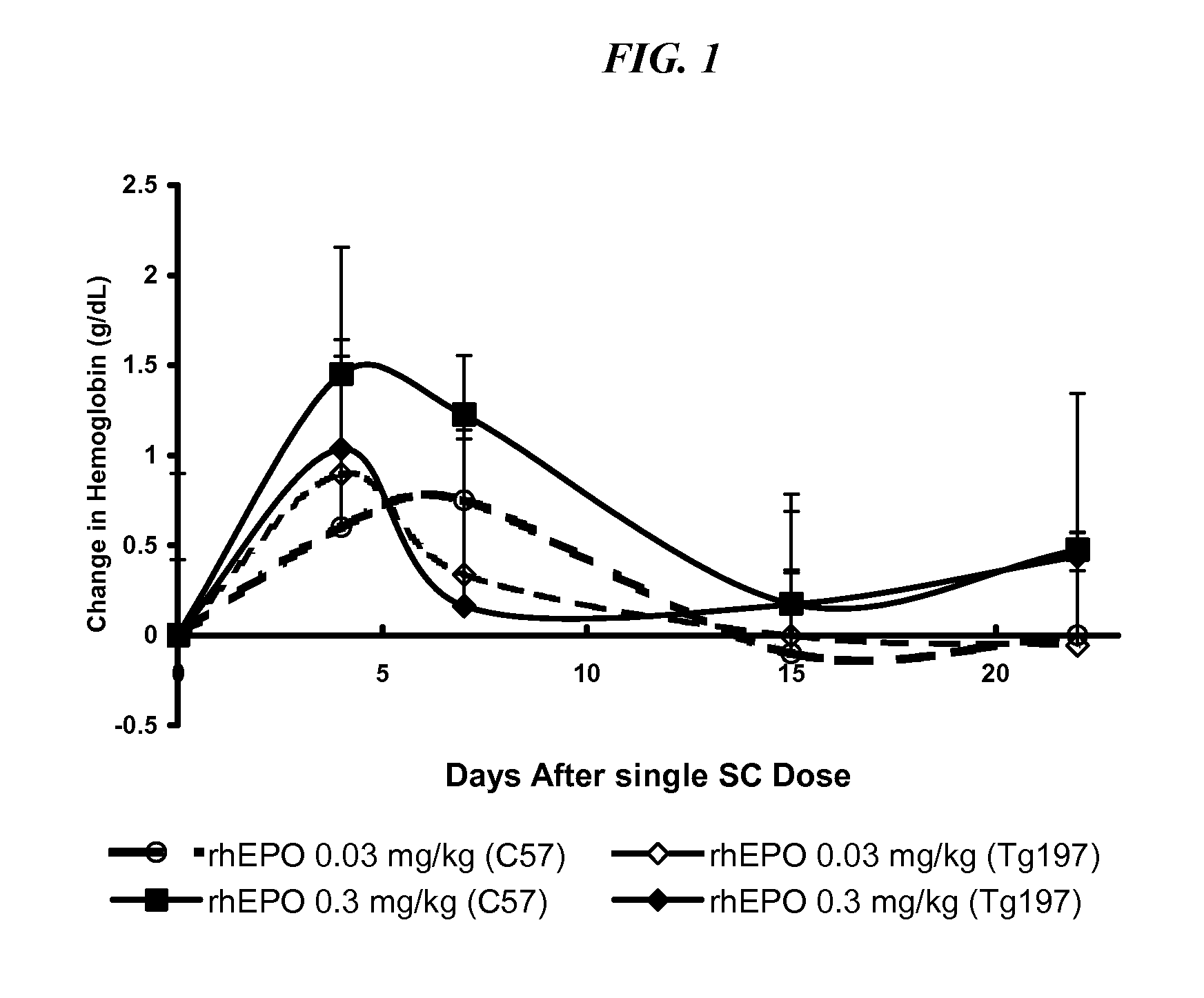
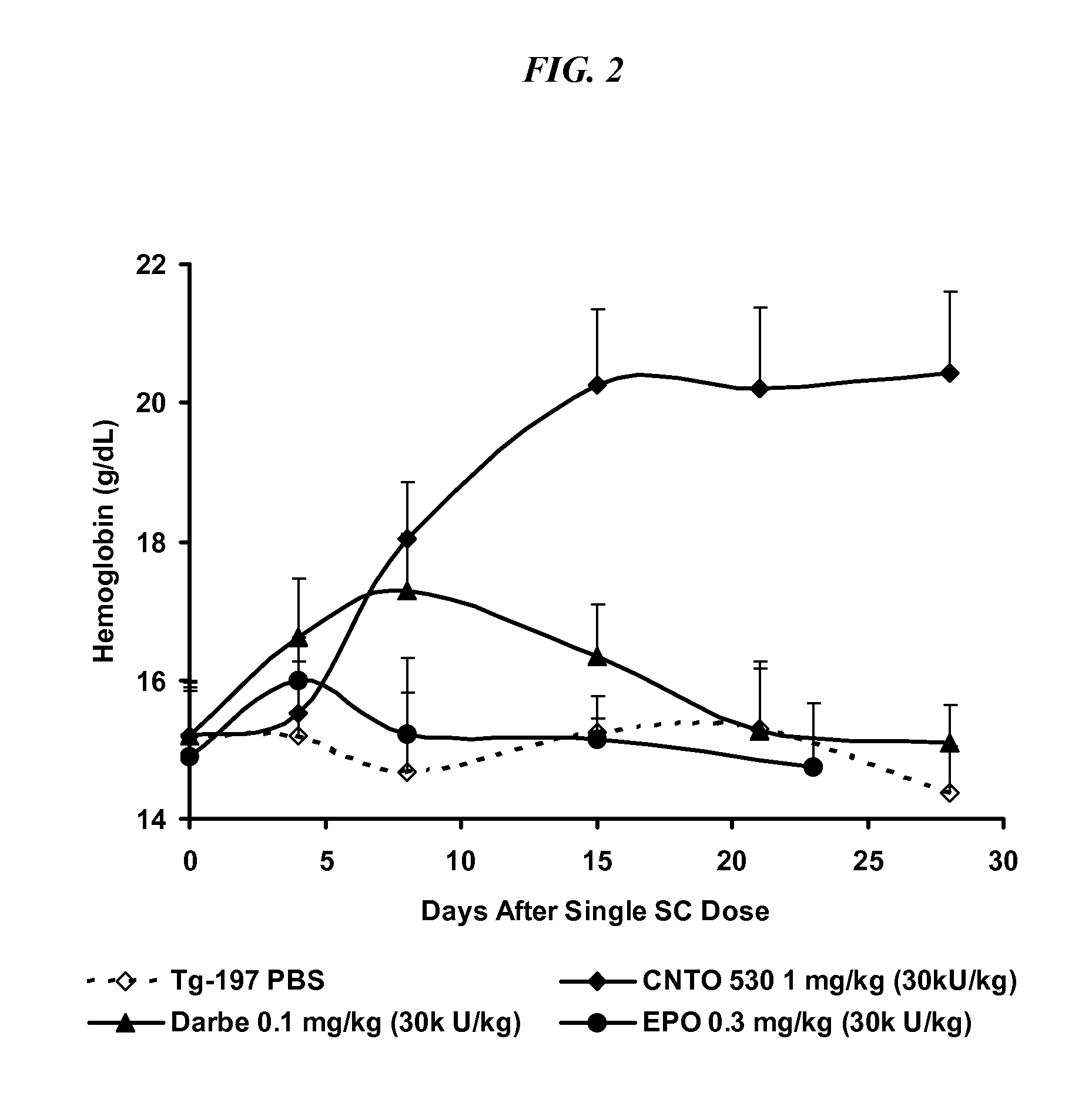
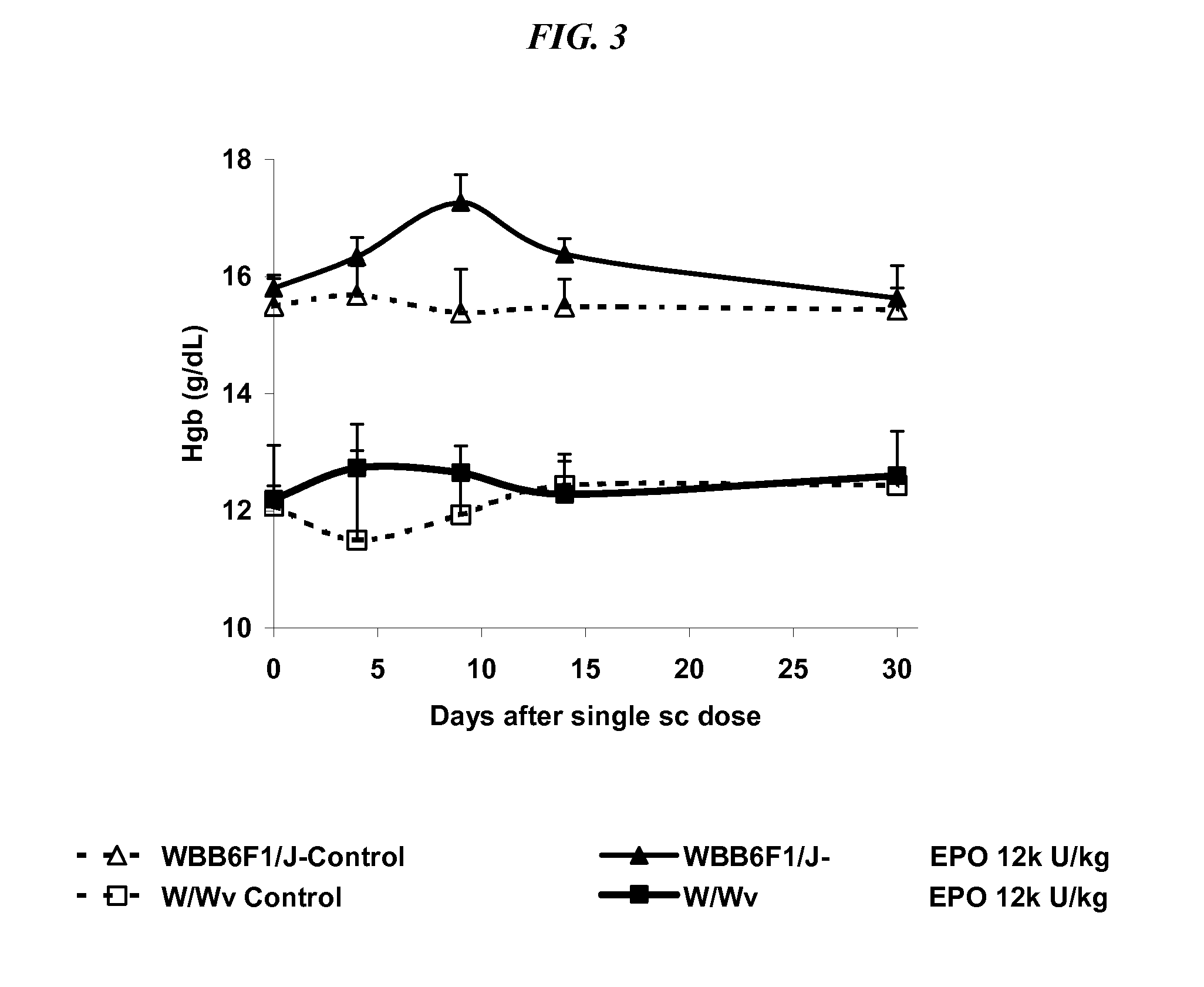
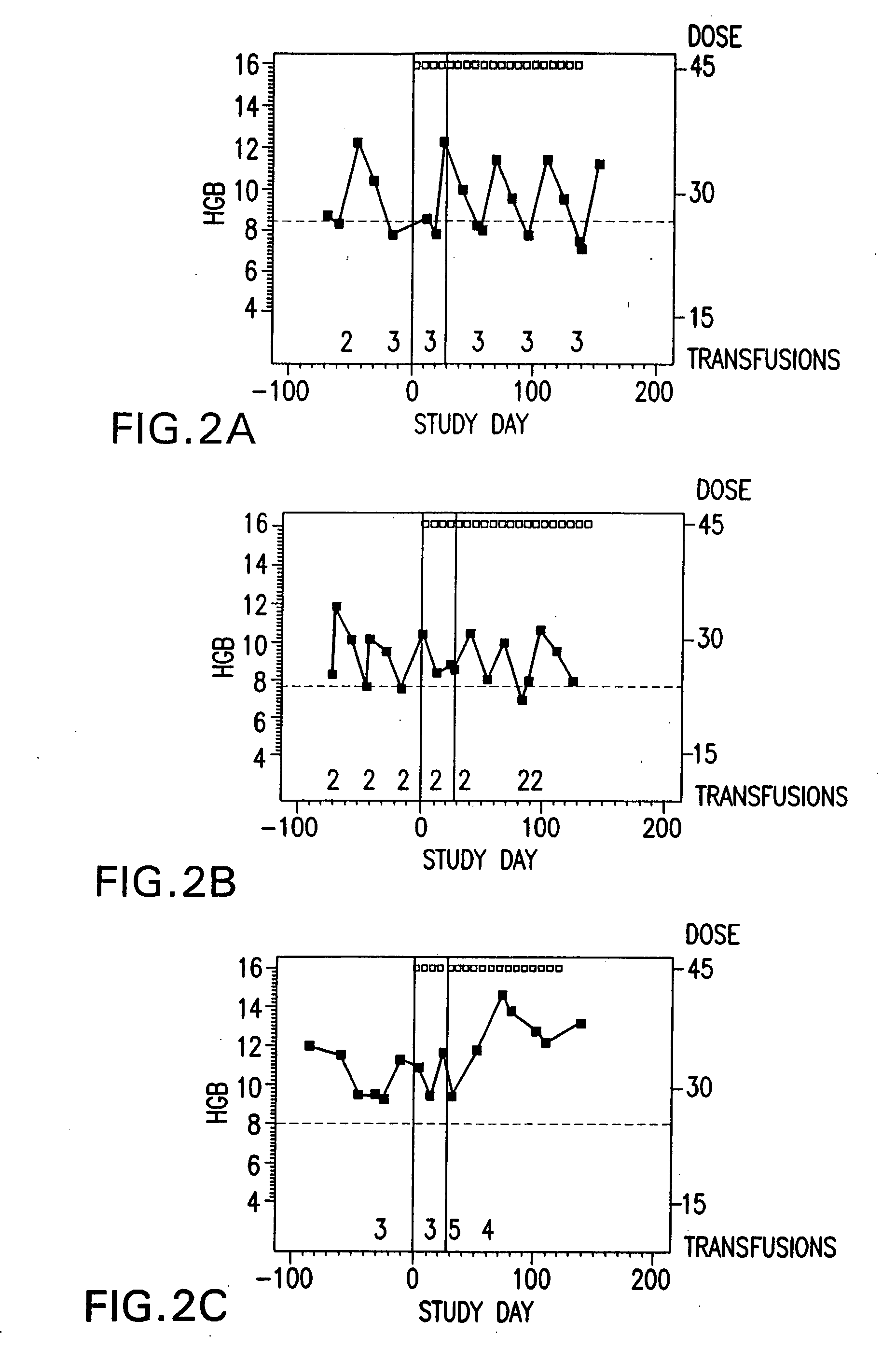
Method of treating erythropoietin hyporesponsive anemias
InactiveUS20100266591A1Peptide/protein ingredientsAntibody ingredientsHemoglobinopathyRed blood cell
The invention relates to methods of using compositions comprising EPO-mimetic peptides to treat anemia. The invention relates to methods of treating disorders characterized by the insufficient amounts of erythrocytes and hemoglobulin in the blood due to myelodysplastic syndrome (MDS) or by hemoglobinopathies, such as alpha- or beta-thalessemia or sickle cell disease.
Owner:CENTOCOR ORTHO BIOTECH
Methods for diagnosing and evaluating treatment of blood disorders
Methods, systems and kits are provided for the clinical staging of blood disorders including myelodysplastic syndrome, myeloproliferative diseases and leukemias by differential analysis of hematologic samples for the distribution of one or more hematopoietic stem or progenitor cell subsets. Additional functional, genetic, gene expression, proteomic or other molecular analyses of the hematopoietic stem and progenitor cells from the patients can also be employed in the staging methods of the invention.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
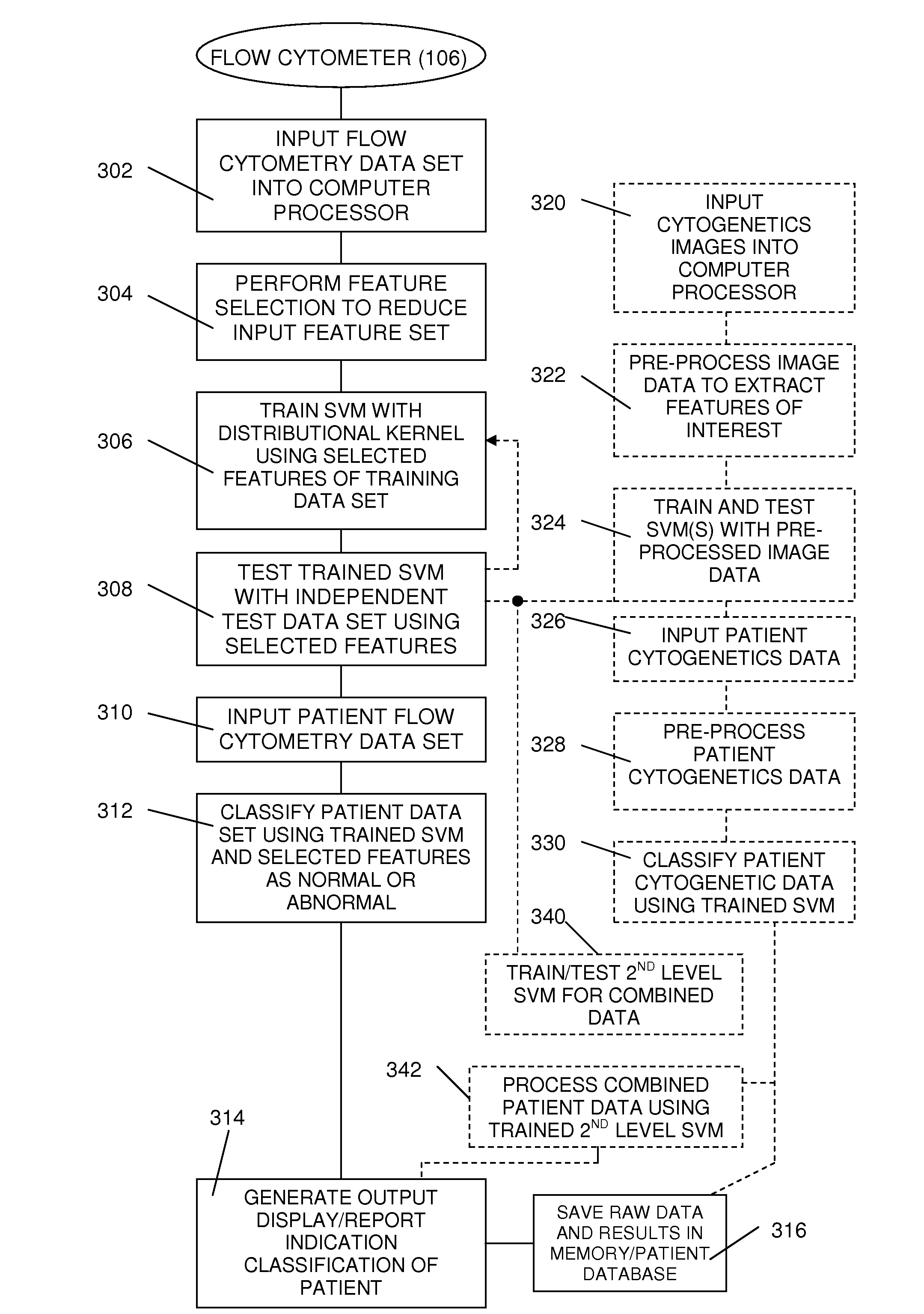
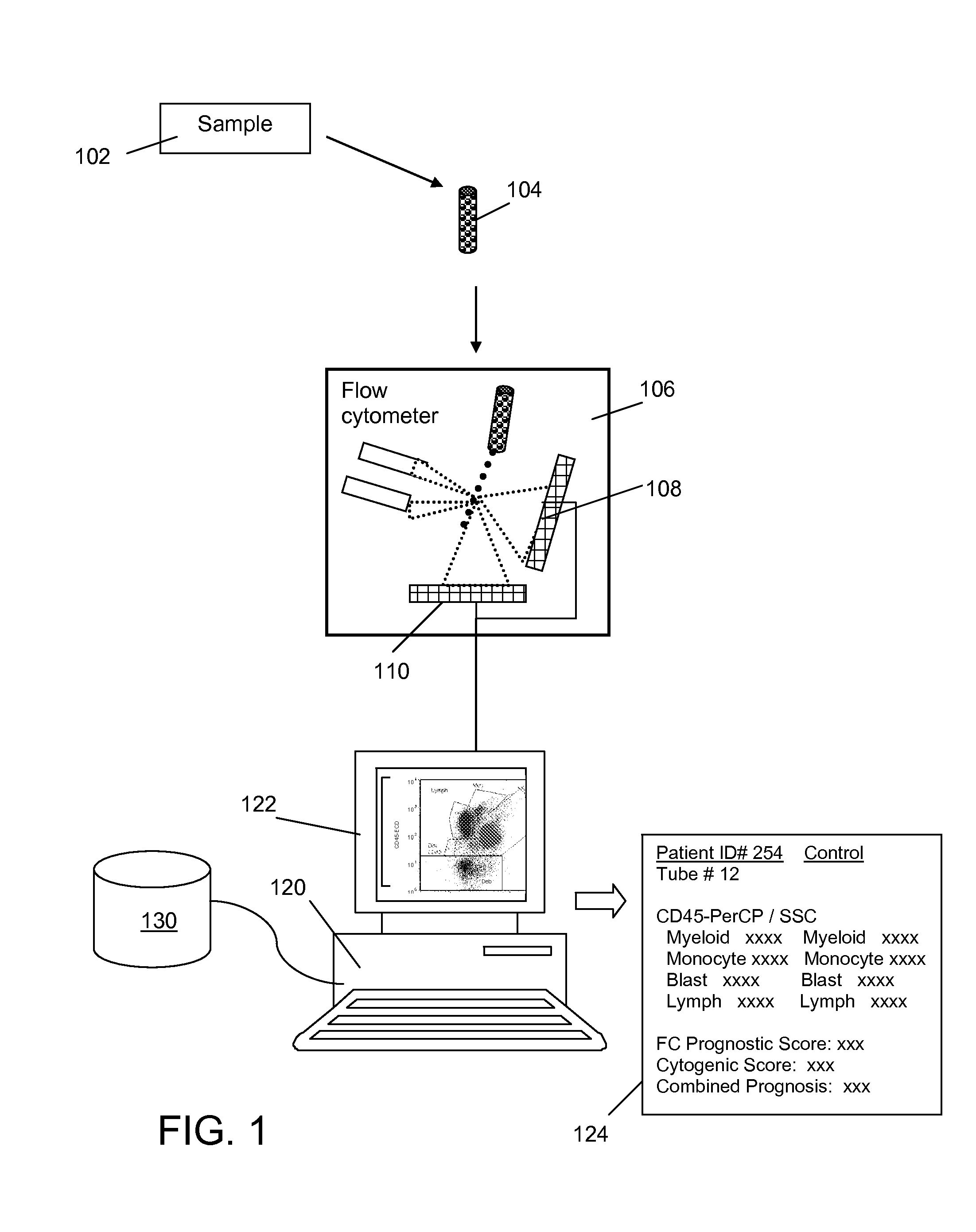
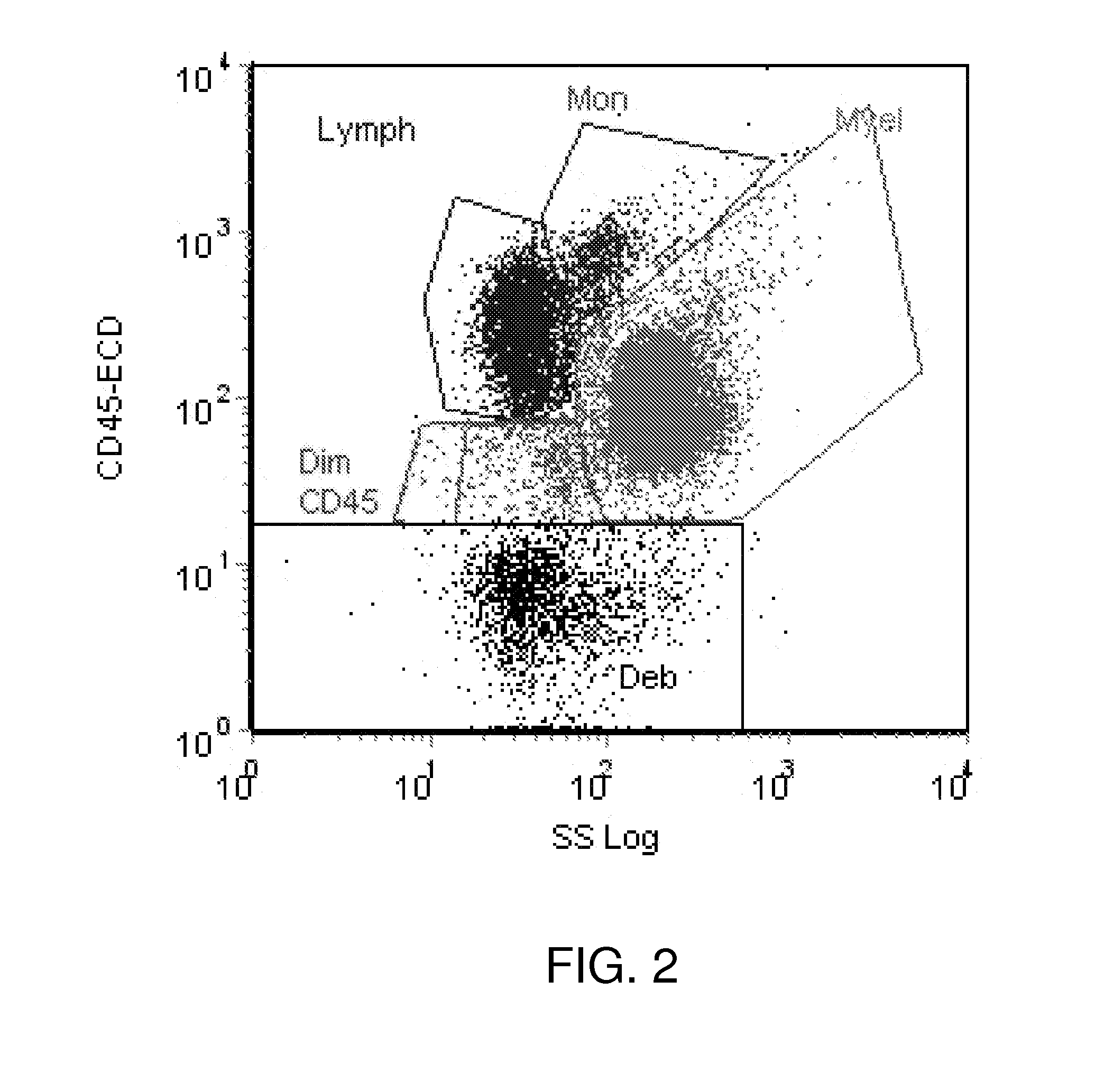
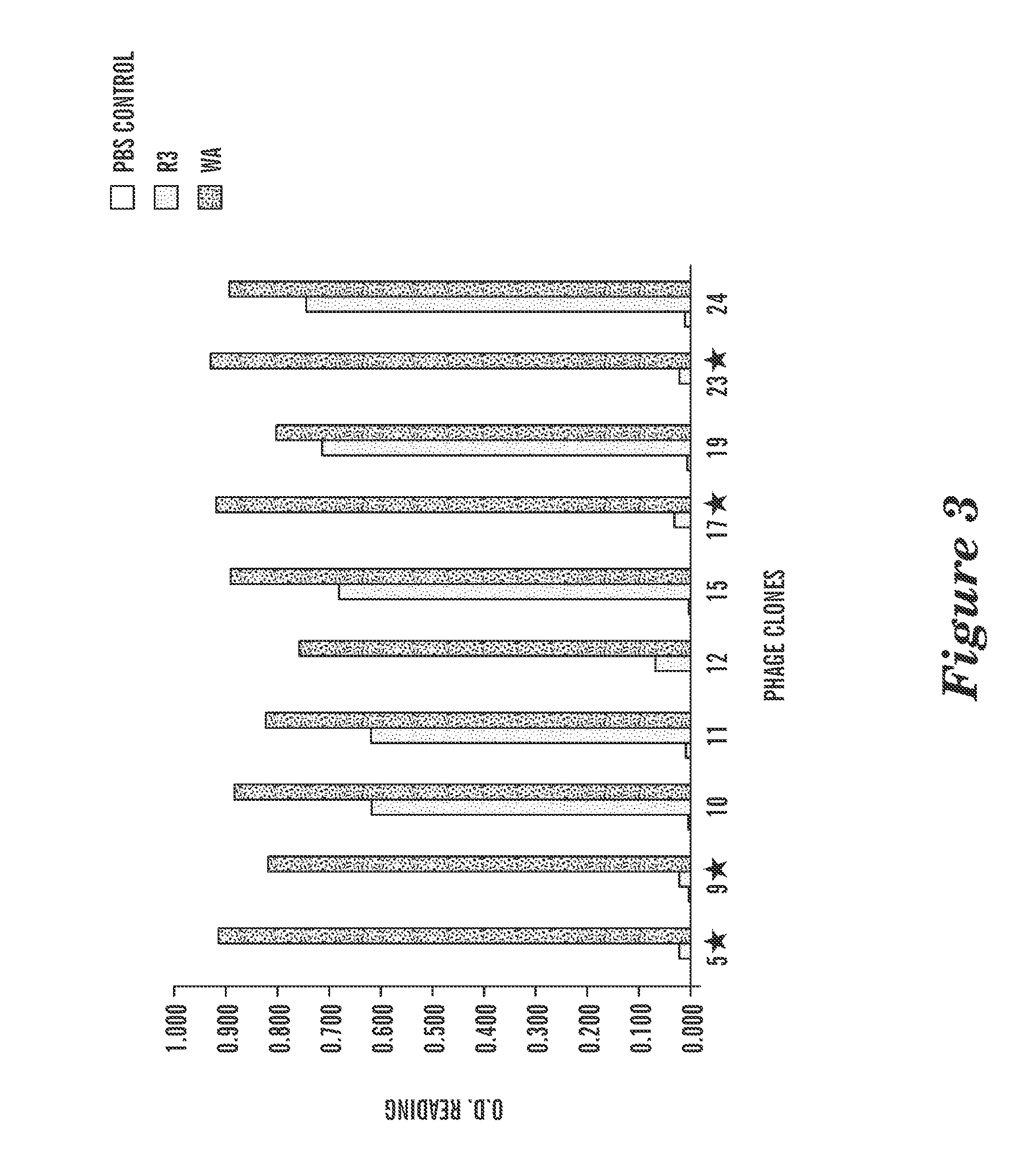
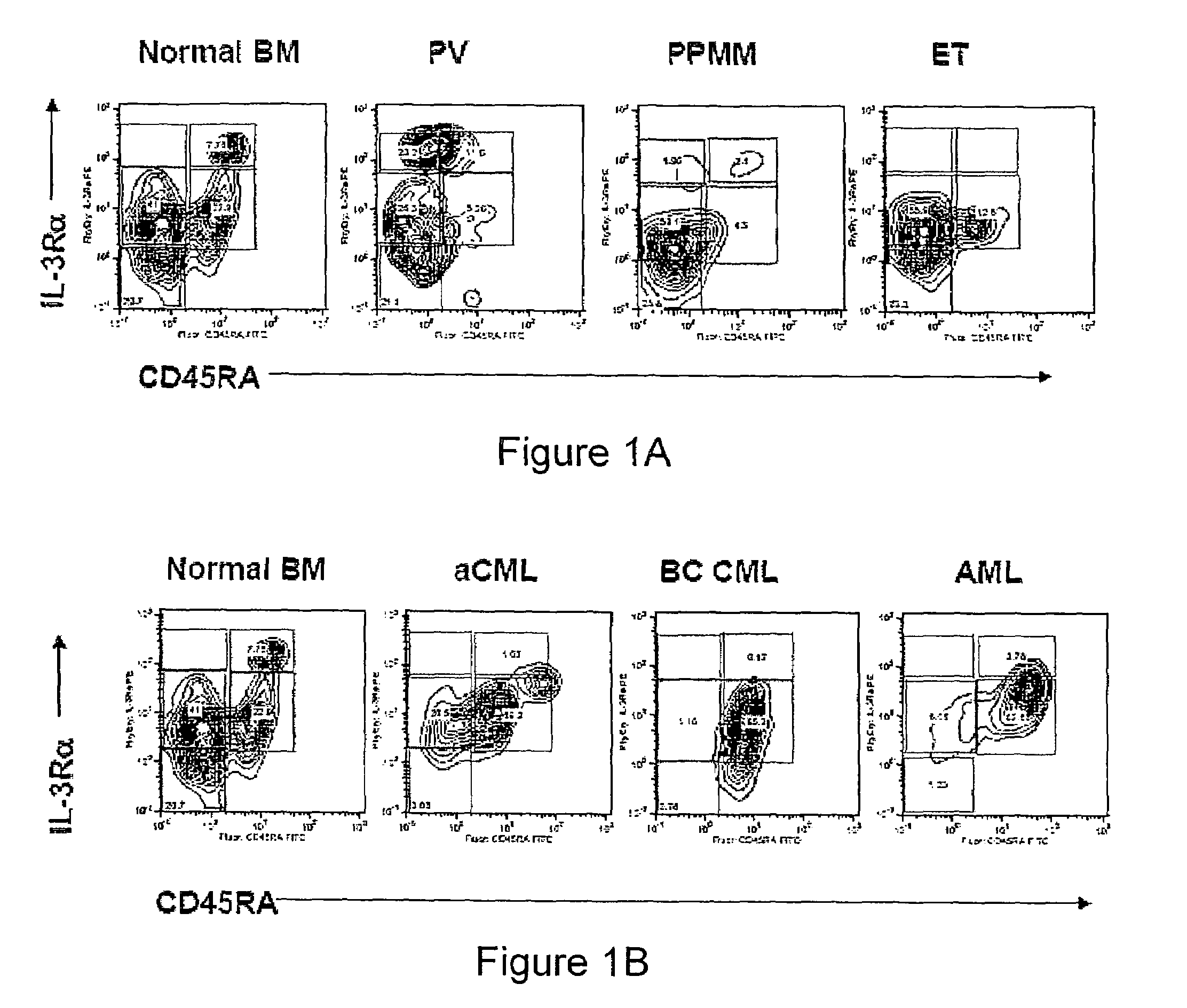
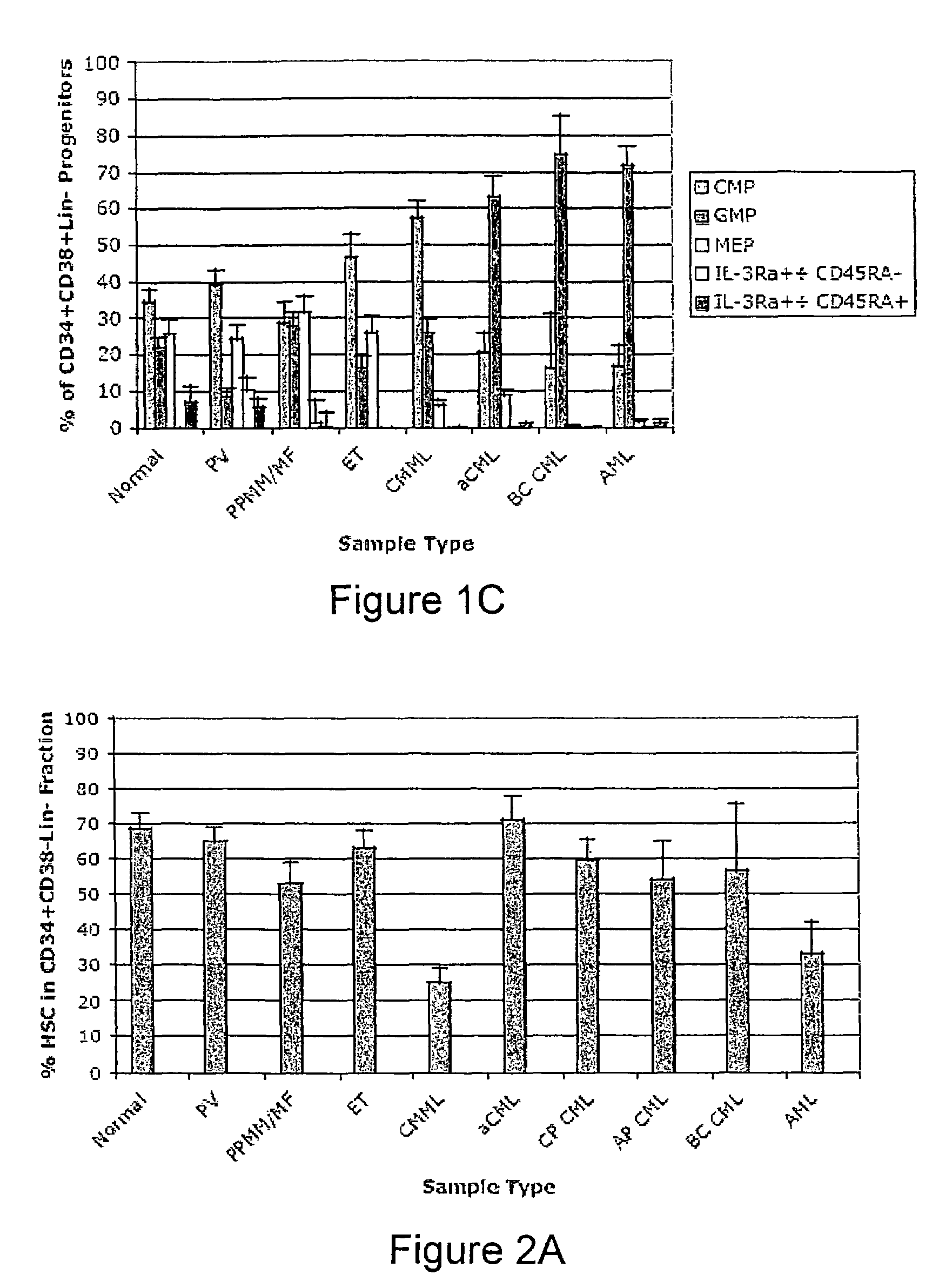
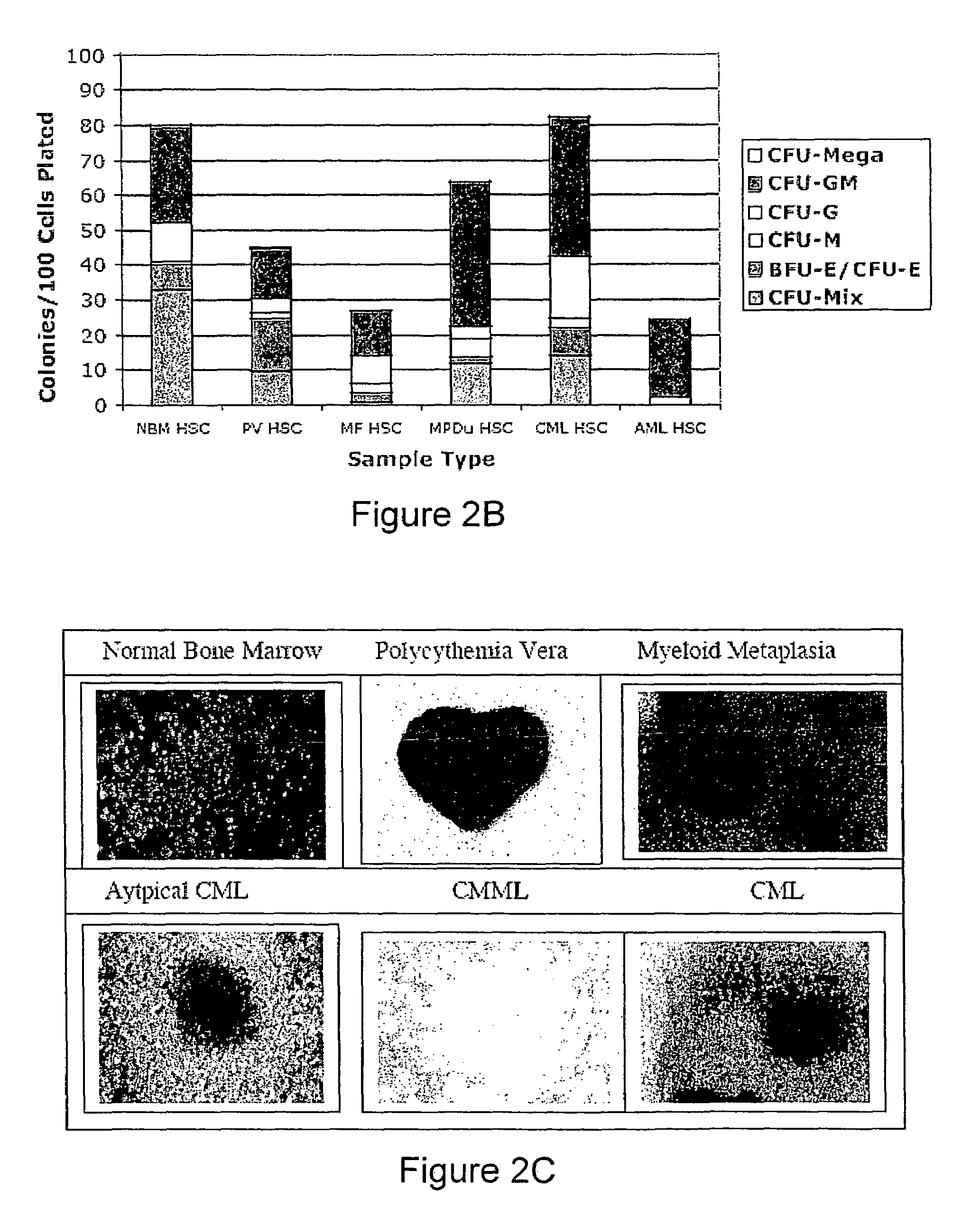
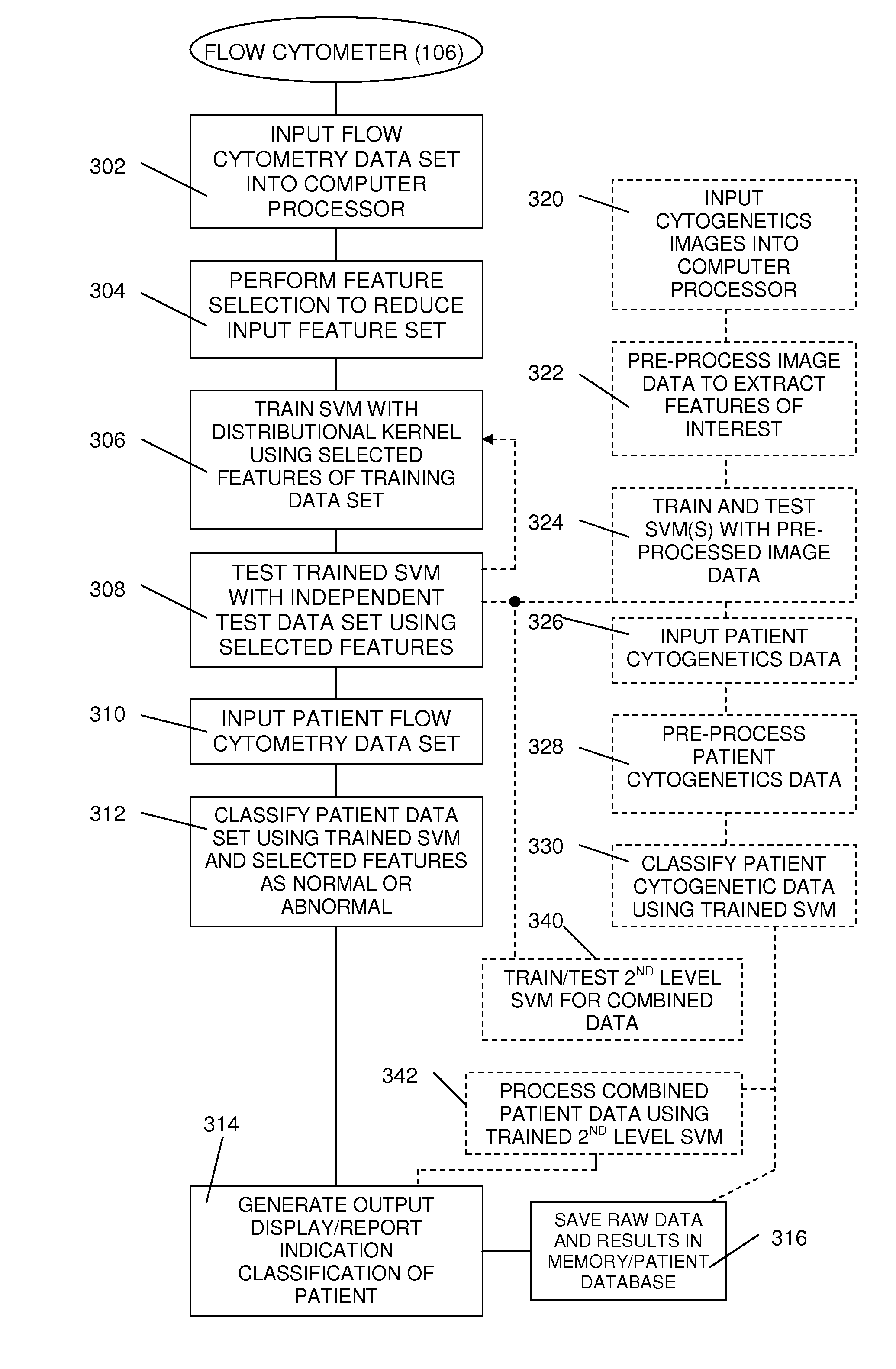
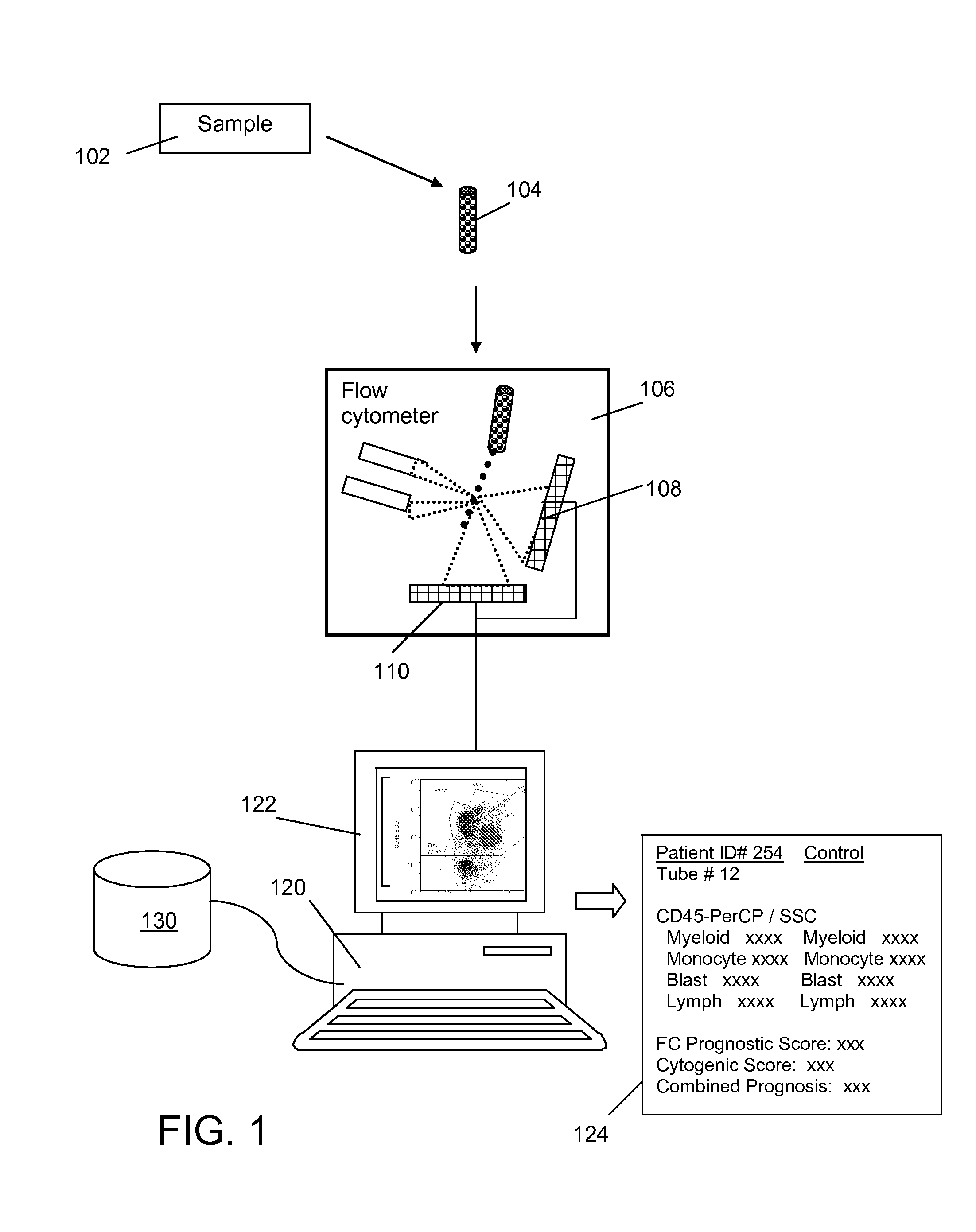
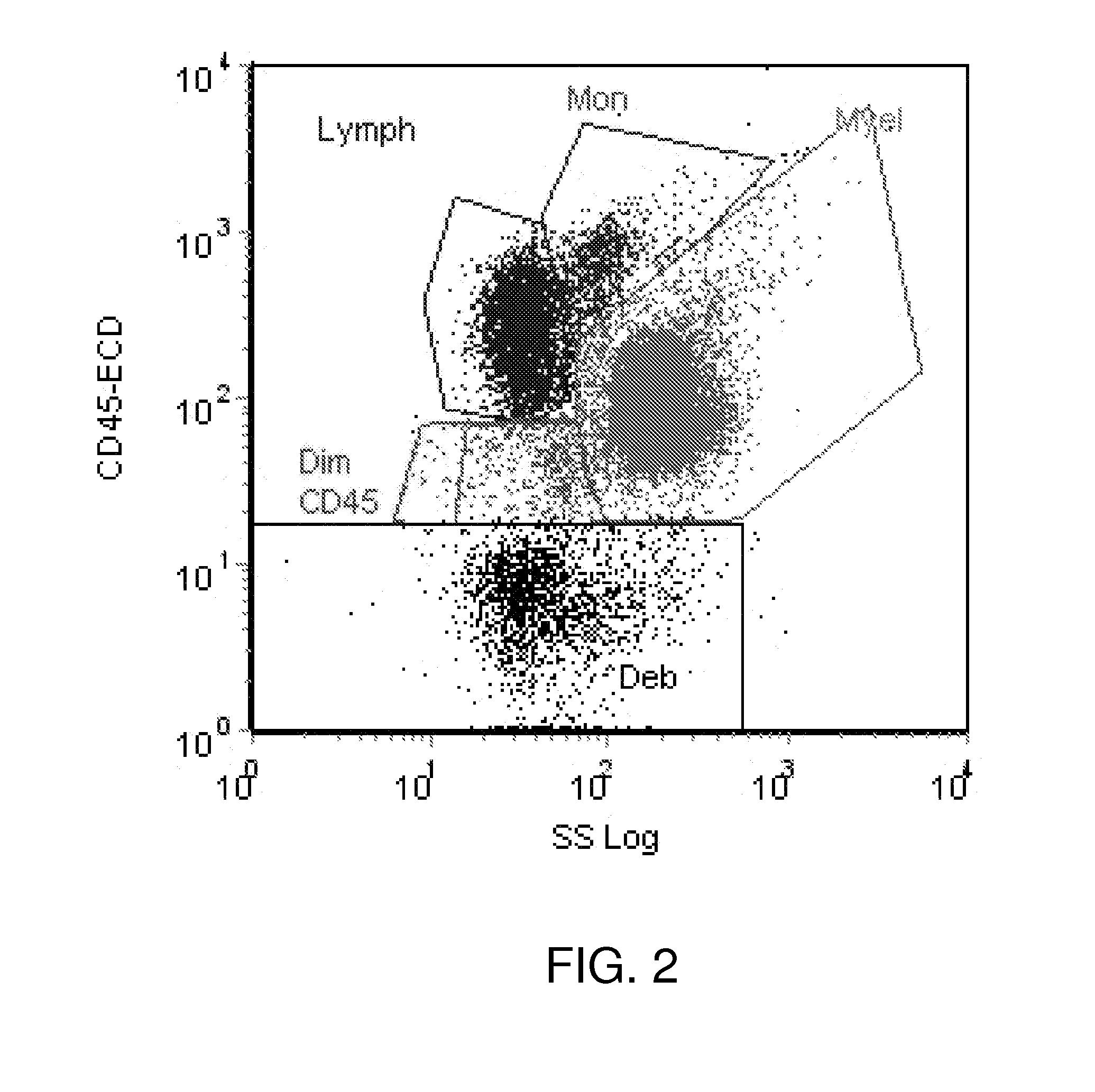
Method and System for Analysis of Flow Cytometry Data Using Support Vector Machines
ActiveUS20090204557A1Digital computer detailsCharacter and pattern recognitionSupport vector machineAlgorithm
An automated method and system are provided for receiving an input of flow cytometry data and analyzing the data using one or more support vector machines to generate an output in which the flow cytometry data is classified into two or more categories. The one or more support vector machines utilizes a kernel that captures distributional data within the input data. Such a distributional kernel is constructed by using a distance function (divergence) between two distributions. In the preferred embodiment, a kernel based upon the Bhattacharya affinity is used. The distributional kernel is applied to classification of flow cytometry data obtained from patients suspected having myelodysplastic syndrome.
Owner:HEALTH DISCOVERY CORP
Methods of using vitamin D compounds in the treatment of myelodysplastic syndromes
InactiveUS20070027120A1Fast concentrationQuick eliminationOrganic active ingredientsBiocideActive agentHigh doses
Owner:WHITEHOUSE MARTHA J +1
T cell receptor-like antibodies specific for a WT1 peptide presented by HLA-A2
The present invention provides antigen binding proteins that specifically bind to Wilms' tumor protein (WT1), including humanized, chimeric and fully human antibodies against WT1, antibody fragments, chimeric antigen receptors (CARs), fusion proteins, and conjugates thereof. The antigen binding proteins and antibodies bind to HLA-A0201-restricted WT1 peptide. Such antibodies, fragments, fusion proteins and conjugates thereof are useful for the treatment of WT1 associated cancers, including for example, breast cancer, ovarian cancer, prostate cancer, chronic myelocytic leukemia, multiple myeloma, acute lymphoblastic leukemia (ALL), acute myeloid / myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). In more particular embodiments, the anti-WT1 / A antibodies may comprise one or more framework region amino acid substitutions designed to improve protein stability, antibody binding and / or expression levels.
Owner:EUREKA THERAPEUTICS INC +1
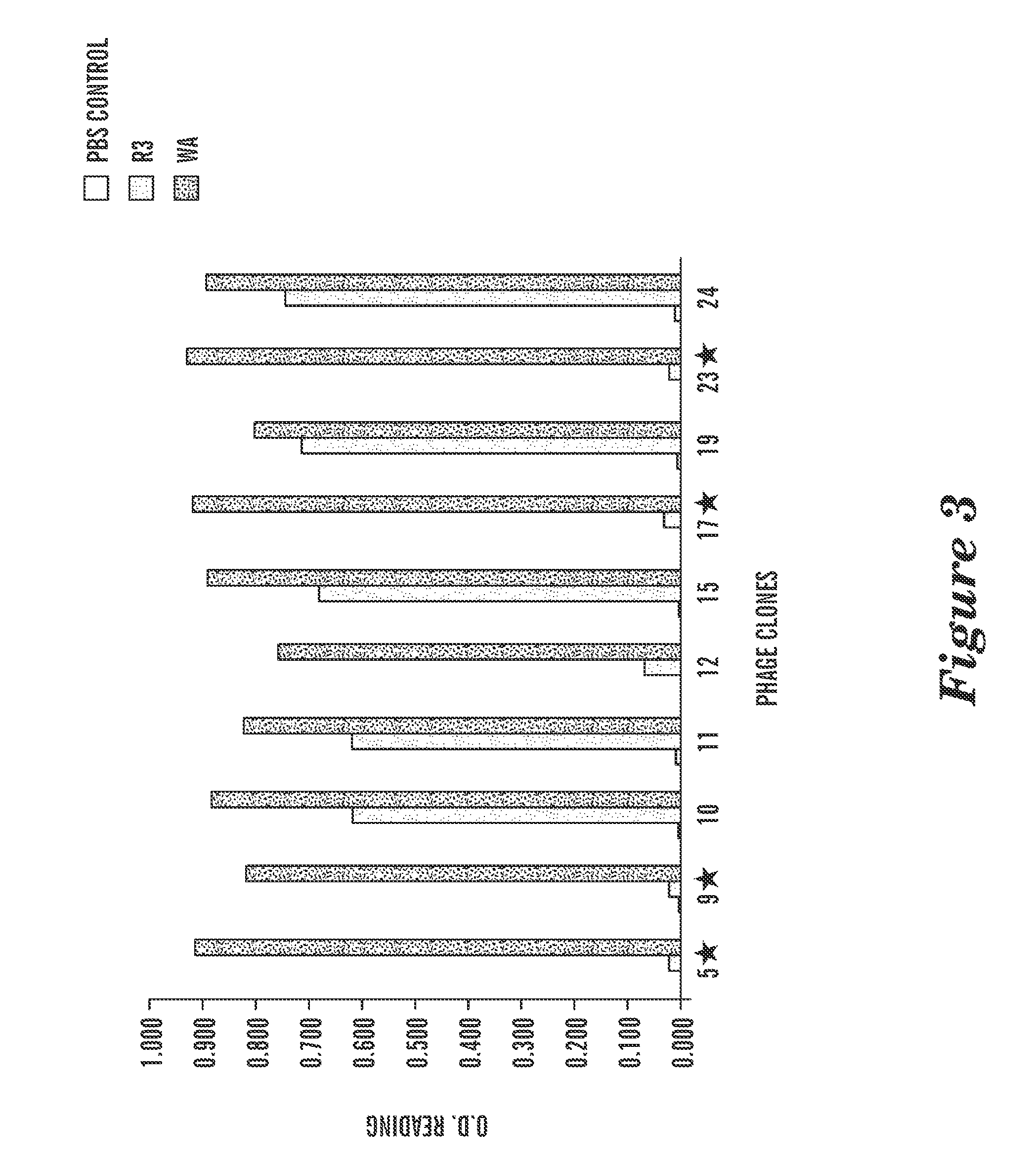
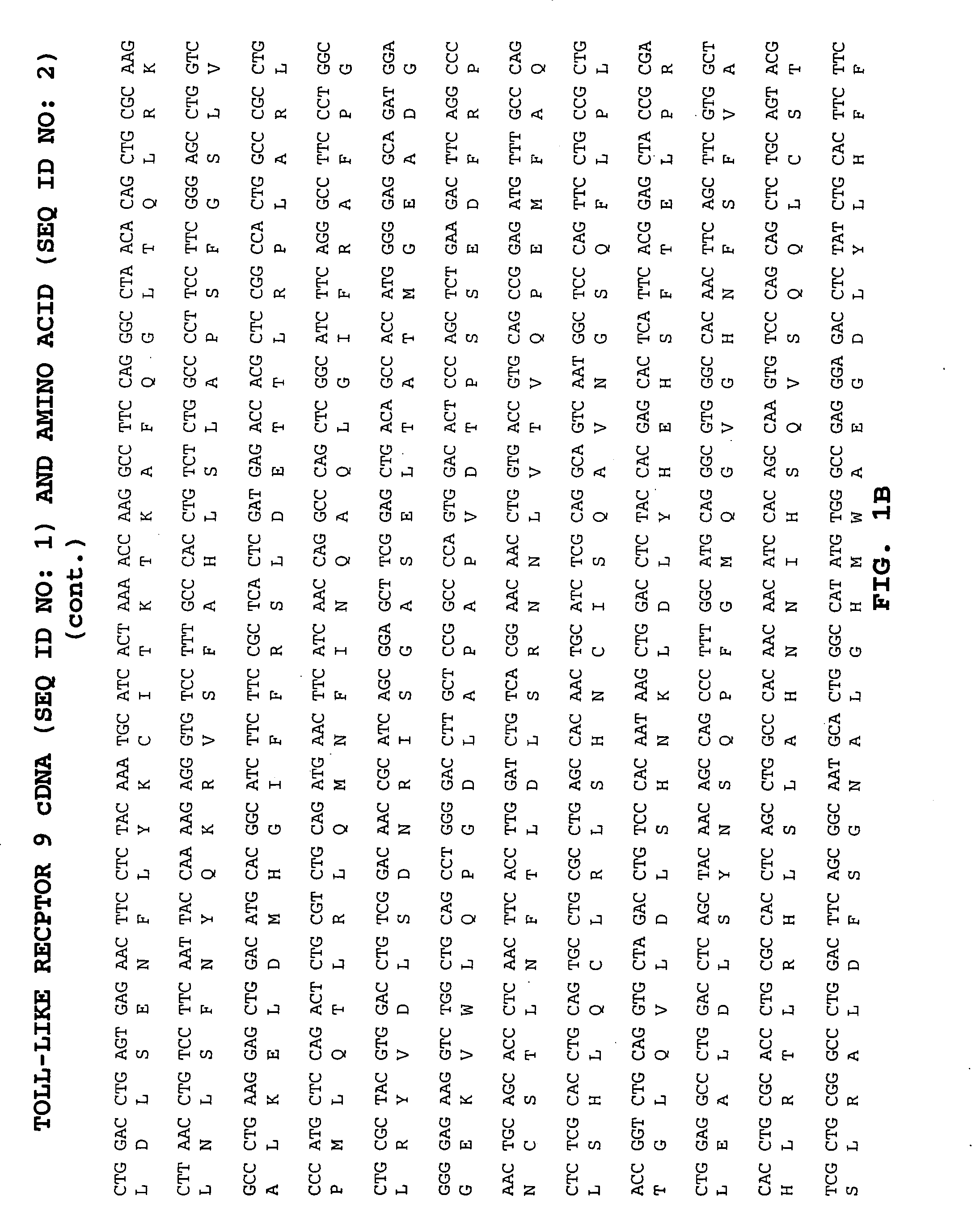
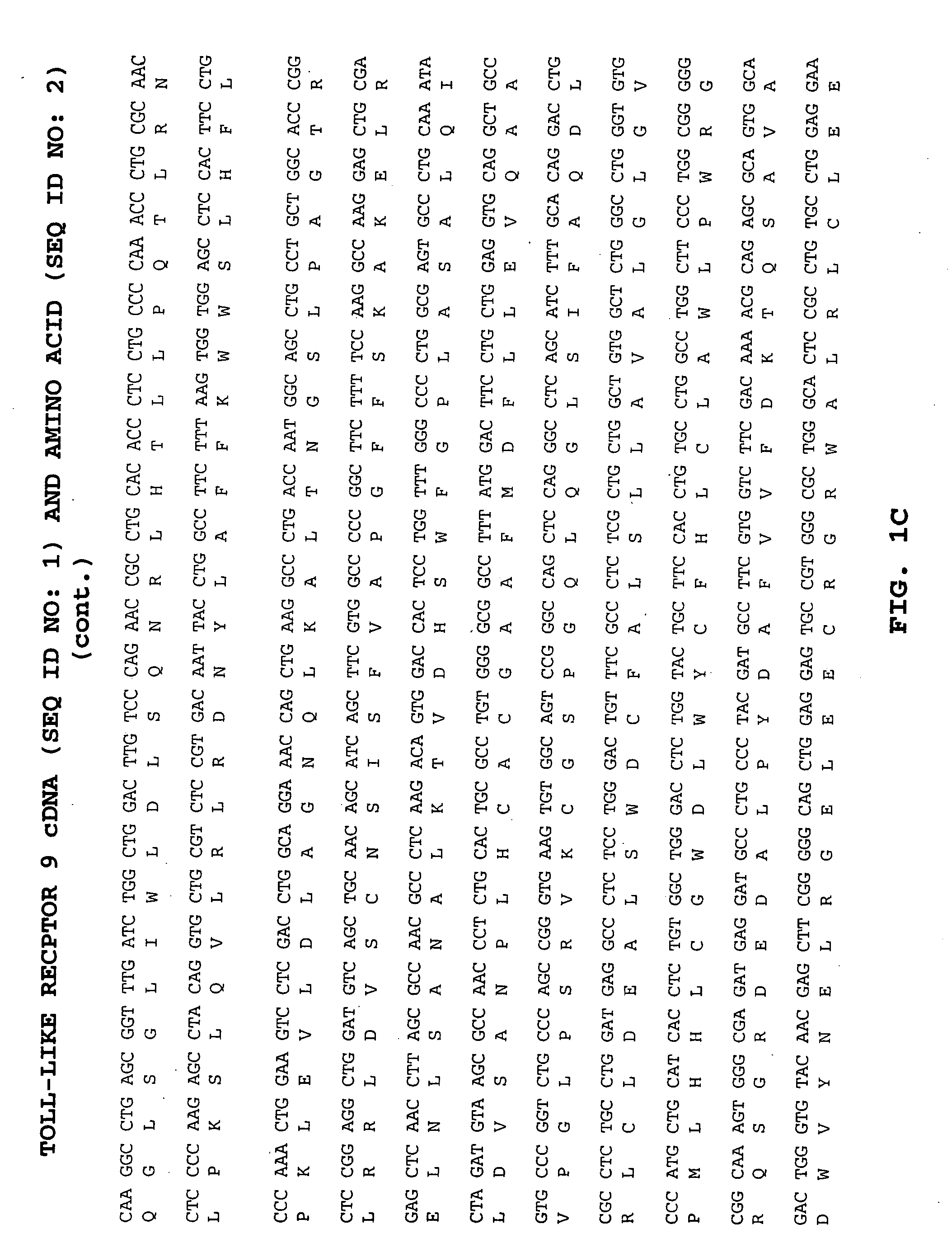
Methods of therapy and diagnosis using targeting of cells that express toll-like receptor proteins
InactiveUS20050281813A1Good effectPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellT cell
Certain cells, including types of cancer cells such as B-cell lymphomas, T cell lymphomas, Hodgkin's disease and myeloid leukemias, are capable of expressing Toll-like Receptor 9 (TLR9) or Toll-like Receptor 10 (TLR10) mRNA. Immunotargeting using TLR9 or TLR10 polypeptides, nucleic acids encoding for TLR9 or TLR10 polypeptides and anti-TLR9 or anti-TLR10 antibodies provides a method of killing or inhibiting that growth of cancer cells that express the TLR9 or TLR10 protein. Methods of immunotherapy and diagnosis of disorders associated with TLR9 or TLR10 protein-expressing cells, such as B-cell lymphoma, T cell lymphoma, acute myeloid leukemia, Hodgkin's disease, B cell leukemia, chronic lymphocytic leukemia, chronic myelogenous leukemia and myelodysplastic syndromes, are described.
Owner:NUVELO INC
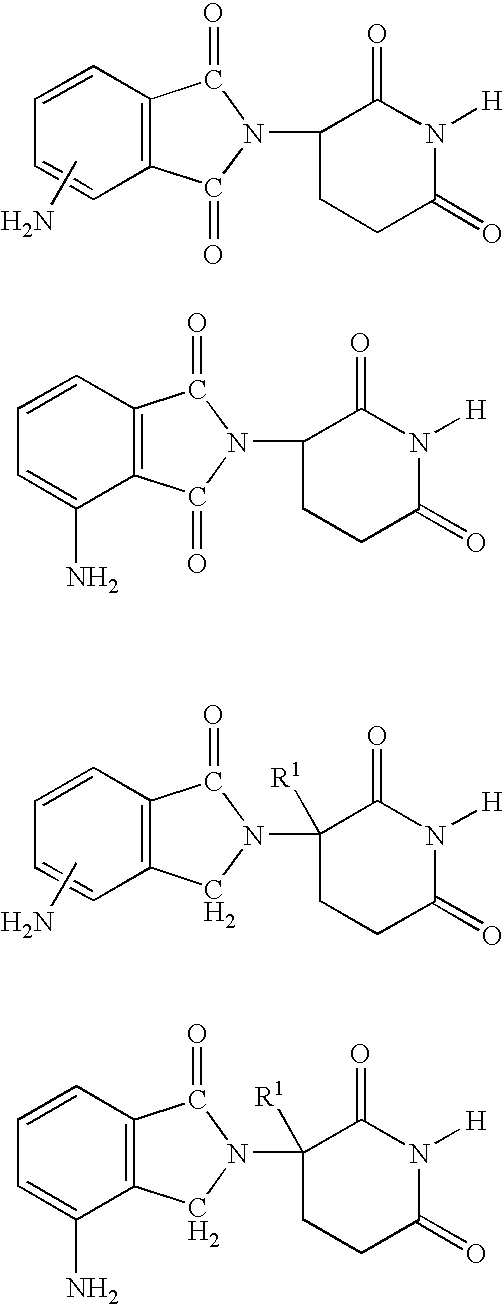
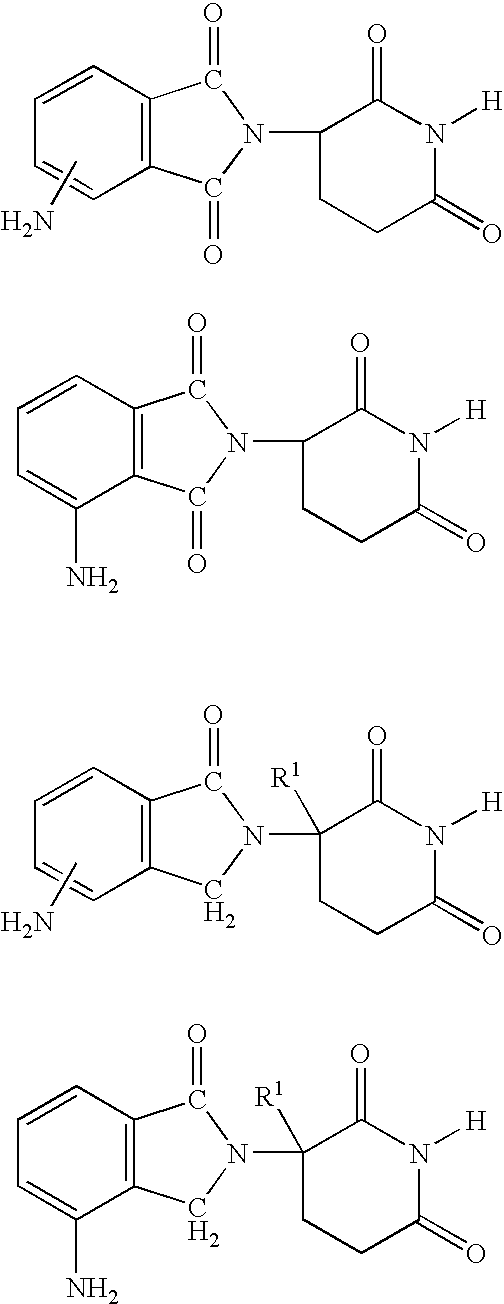
7-Amido-isoindolyl compounds and their pharmaceutical uses
The invention encompasses 7-amido-isoindolyl compounds and methods of using these compounds and compositions in mammals for treatment, prevention or management of various diseases and disorders. Examples inlcude, but are not limited to, cancer, inflammatory bowel disease and myelodysplastic syndrome.
Owner:CELGENE CORP
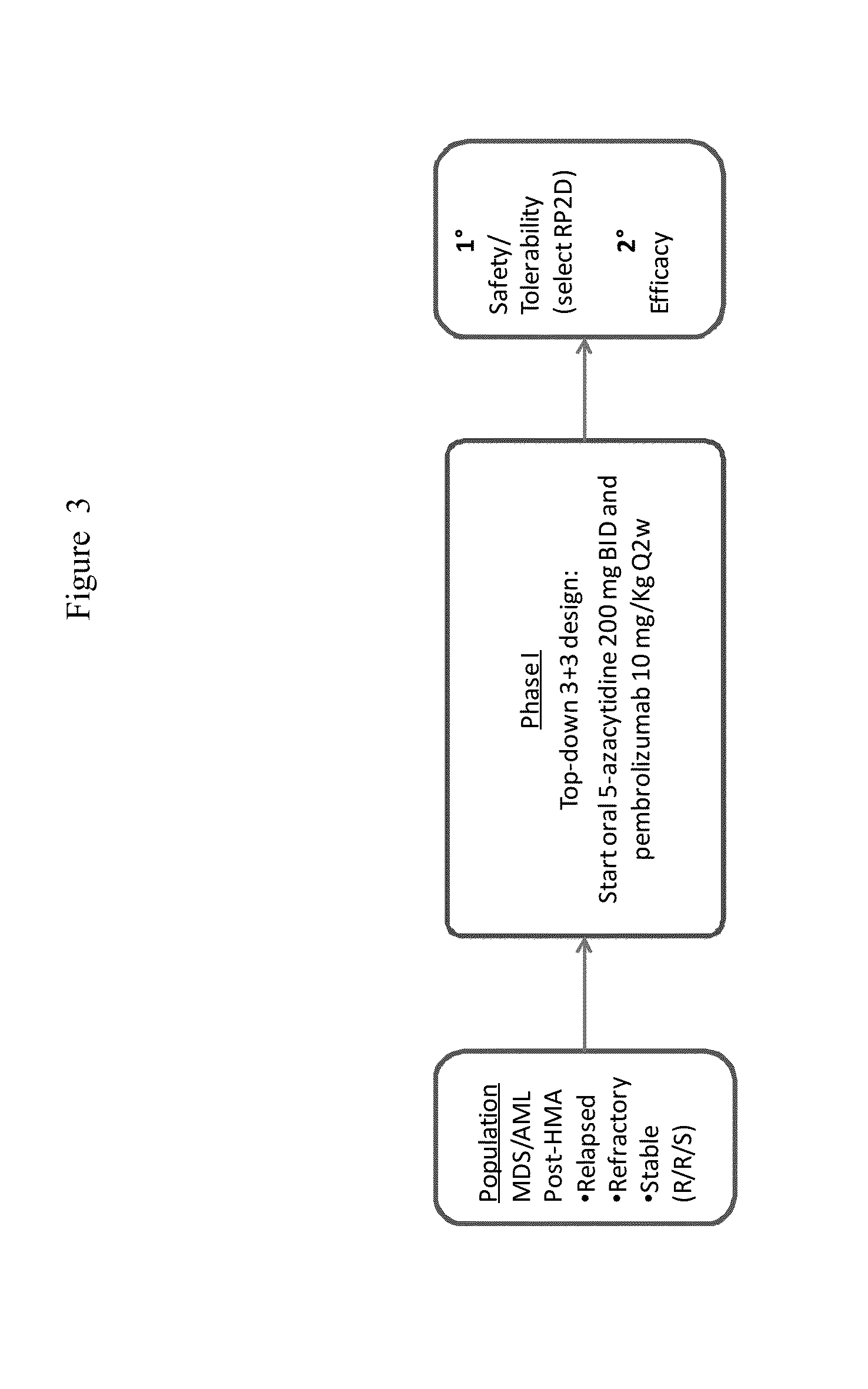
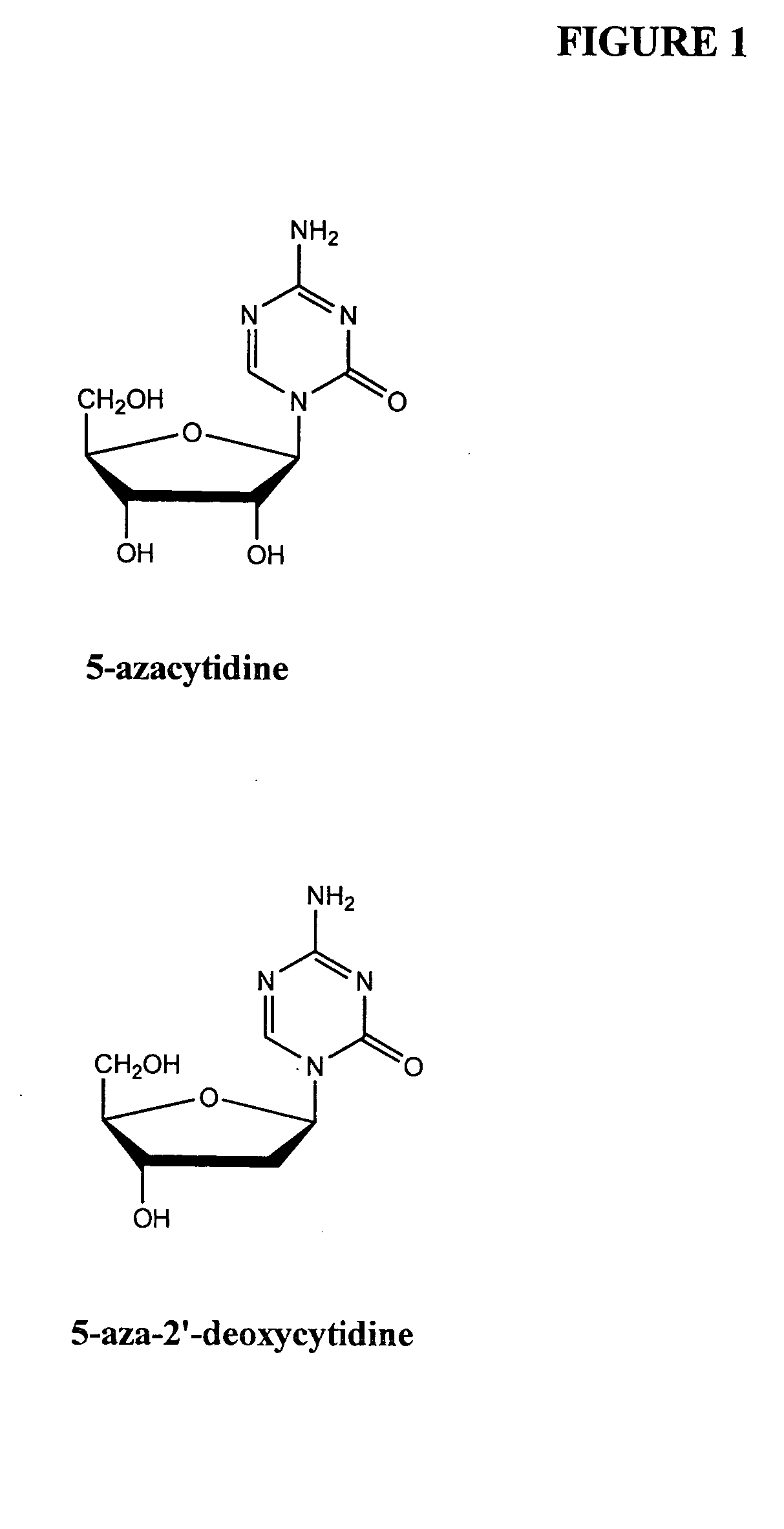
Methods for treating a disease or disorder using oral formulations of cytidine analogs in combination with an Anti-pd1 or Anti-pdl1 monoclonal antibody
InactiveUS20160067336A1Improve survivalOrganic active ingredientsAntibody ingredientsDiseaseMonoclonal antibody
The present disclosure provides methods of treating diseases or disorders with oral cytidine analogs (e.g., 5-azacytidine) in combination with anti-PD1 / anti-PDL1 antibodies (e.g., pembrolizumab or durvalumab). The diseases or disorders include, but are not limited to, relapsed or refractory myelodysplastic syndromes, acute myeloid leukemia, ovarian cancer, or non-small cell lung cancer.
Owner:CELGENE CORP
Methods for treating hematological disorders through inhibition of DNA methylation and histone deacetylase
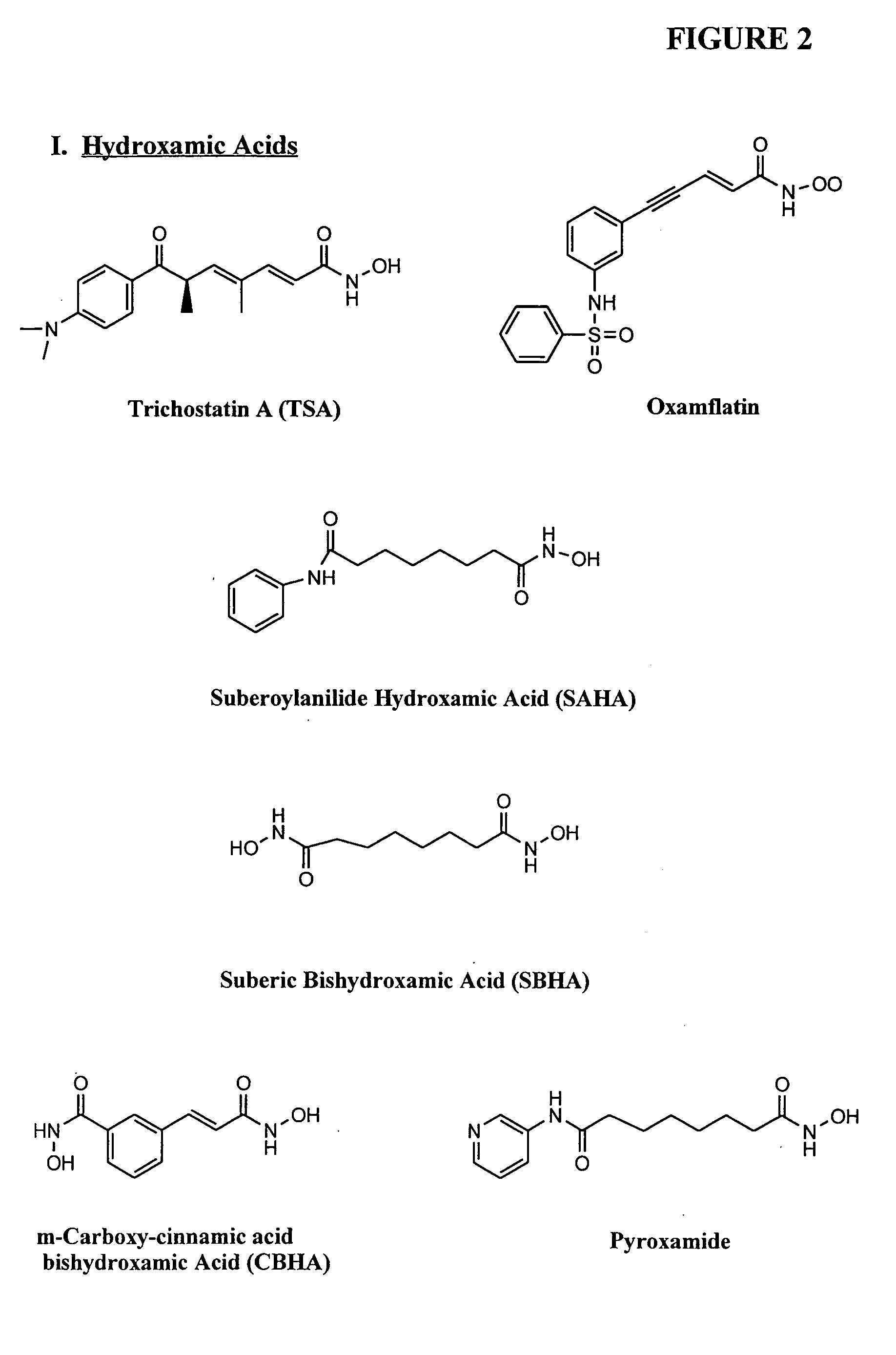
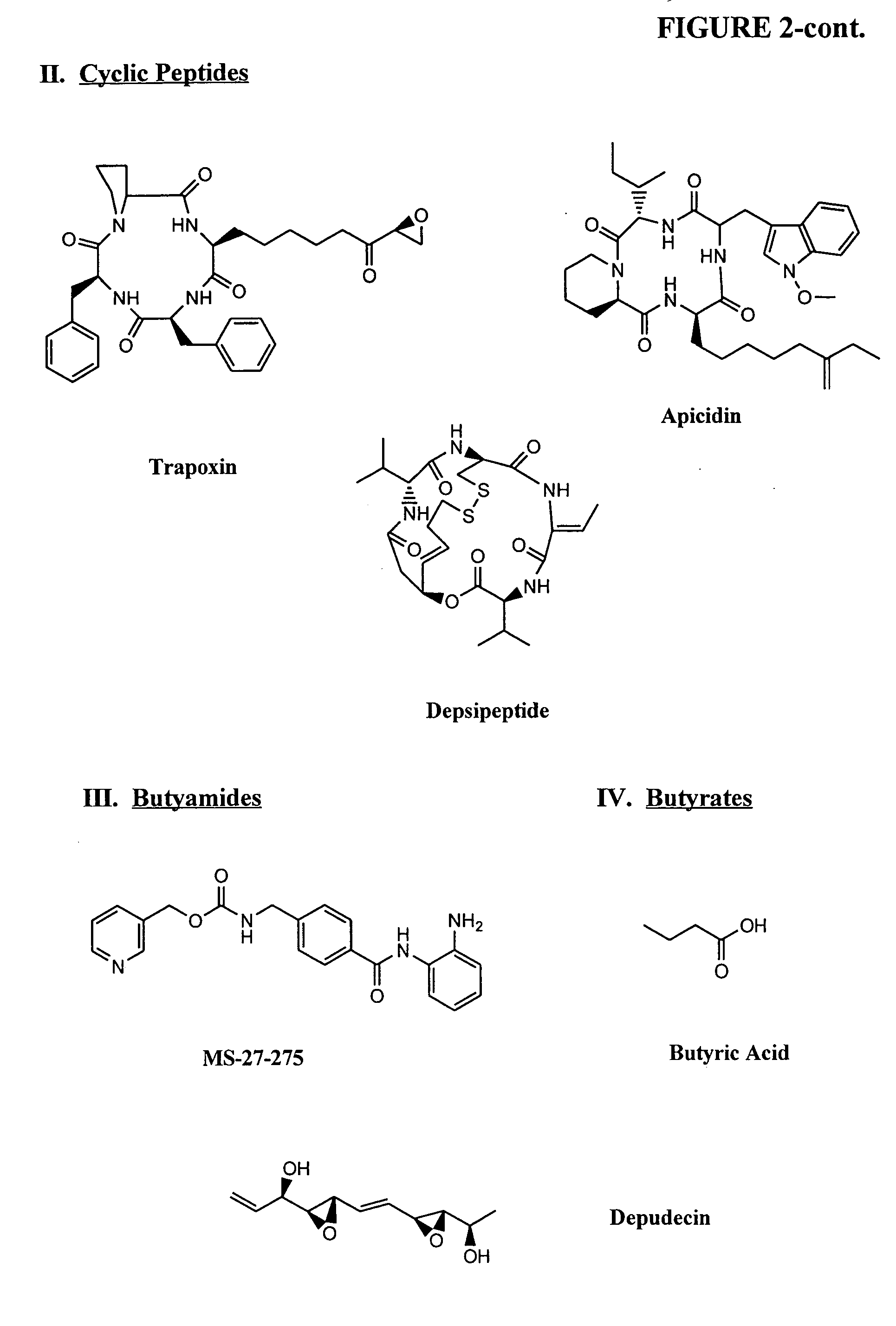
InactiveUS20050159347A1Address bad outcomesReduce dosageAntibacterial agentsBiocideCyclic peptideHydroxamic acid
Methods are provided for treating hematological disorders by inhibition of DNA hypomethylation and histone deacetylase. Such disorders include, for example, acute promyelocytic leukemia, acute lymphoblastic leukemia, chronic myelogenous leukemia, myelodysplastic syndromes, and sickle cell anemia. The methods comprise: administering to a patient suffering from the disease a therapeutically effective amount of a DNA methylation inhibitor such as a cysteine analog such as decitabine, in combination with an effective amount of histone deacetylase inhibitor such as hydroxamic acid, cyclic peptide, benzamide, butyrate, and depudecin.
Owner:SUPERGEN
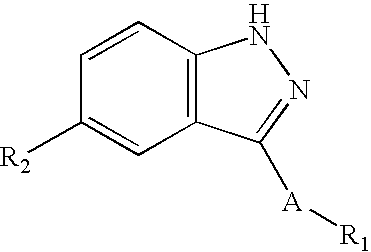
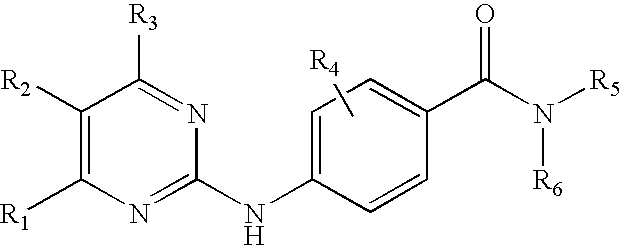
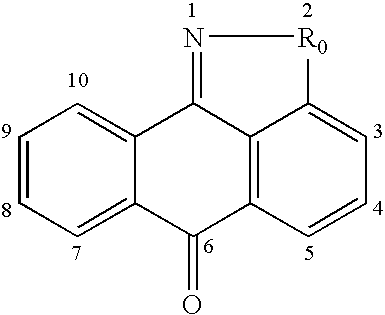
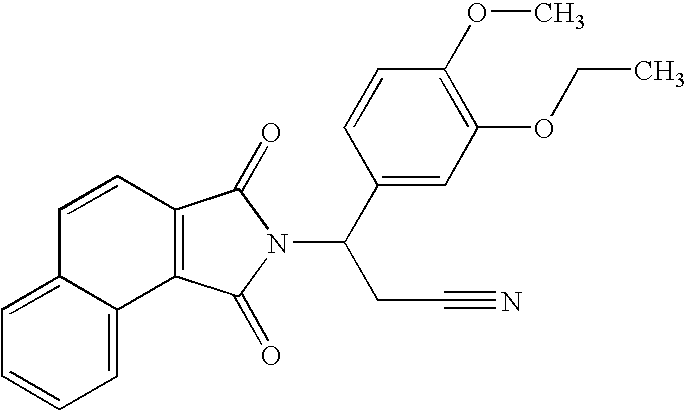
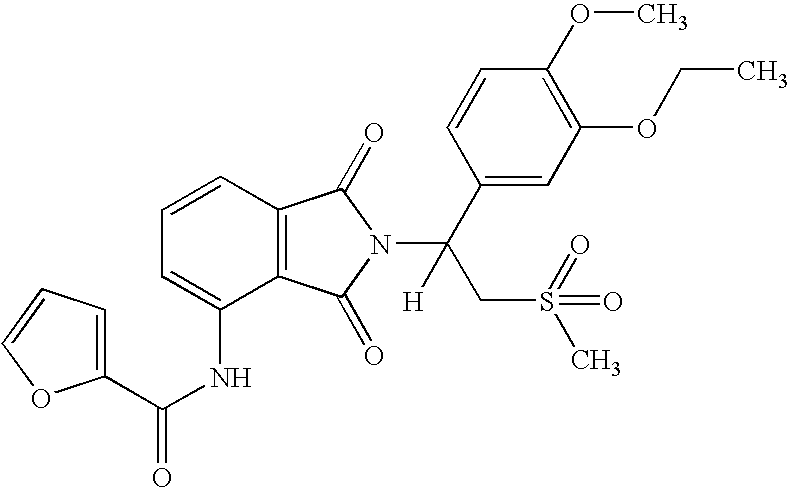
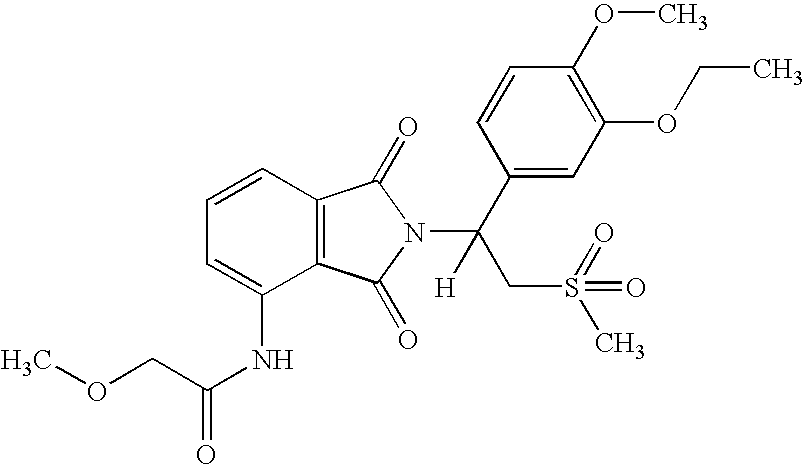
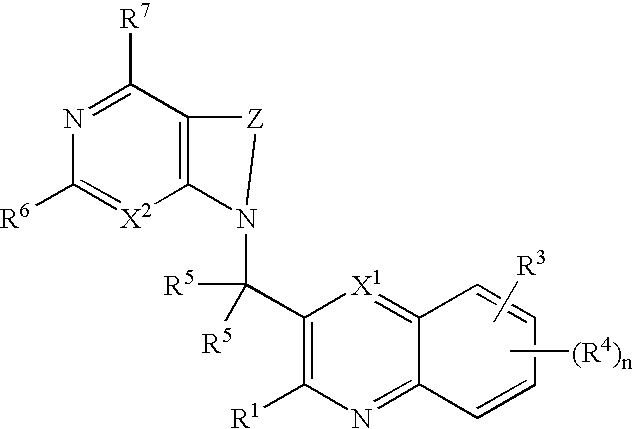
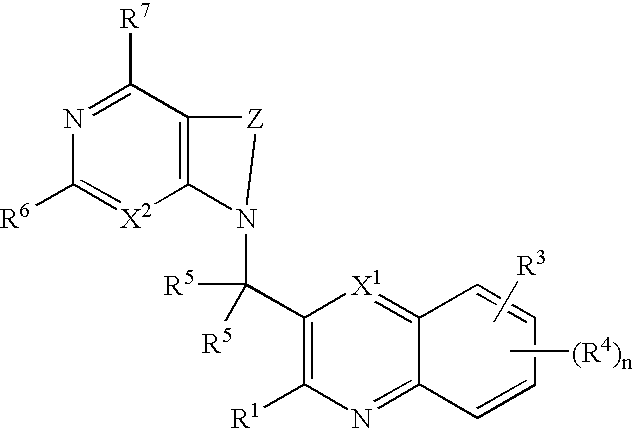
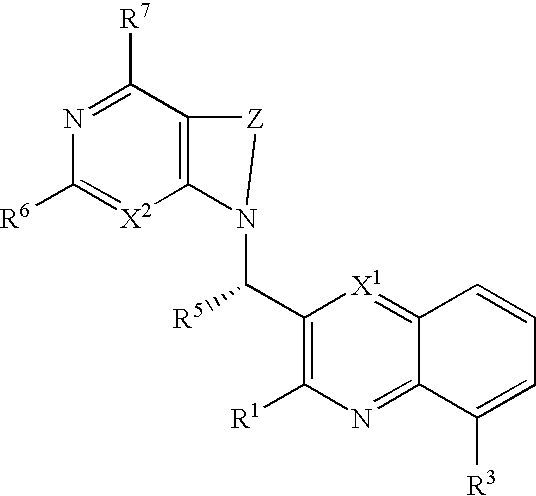
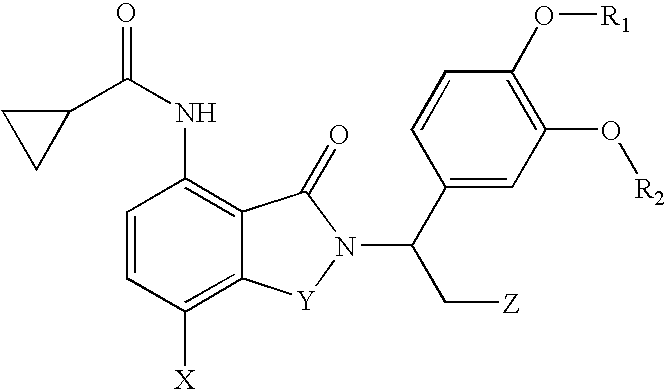
Substituted pyrazolo[3,4-d]pyrimidines as PI3K inhibitors
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110δ activity, including but not restricted to leukemias, such as Acute Myeloid leukemia (AML) Myelodysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
Decitabine freeze-dried powder injection
ActiveCN101584670AImprove stabilityLow content of related substancesOrganic active ingredientsPowder deliveryPhosphateFreeze-drying
The invention relates to a decitabine freeze-dried powder injection and a preparing method thereof. The prepared decitabine freeze-dried powder injection is used for treating myelodysplastic syndrome (MDS). The decitabine freeze-dried powder injection contains decitabine, utilizes the mixed solvent composed of the tert-butyl alcohol and the injection water in the preparation process, wherein the concentration of the decitabine in the mixed solvents is 2.5-5 mg / ml; and the volume ratio of the solvents is: 5-50% of tert-butyl alcohol and the balance of injection water. The potassium dihydrogen phosphate and the sodium hydroxide may be added for the pH regulator. The preparation process comprises the following steps: measuring tert-butyl alcohol, adding injection water, potassium dihydrogen phosphate and sodium hydroxide, stirring and mixing evenly, cooling to 2-15 DEG C, heat preserving, adding decitabine, stirring to dissolve, filtering, filling, plugging, disking, freeze-drying, pressing plug, out box, tying and packing after quality test qualification.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
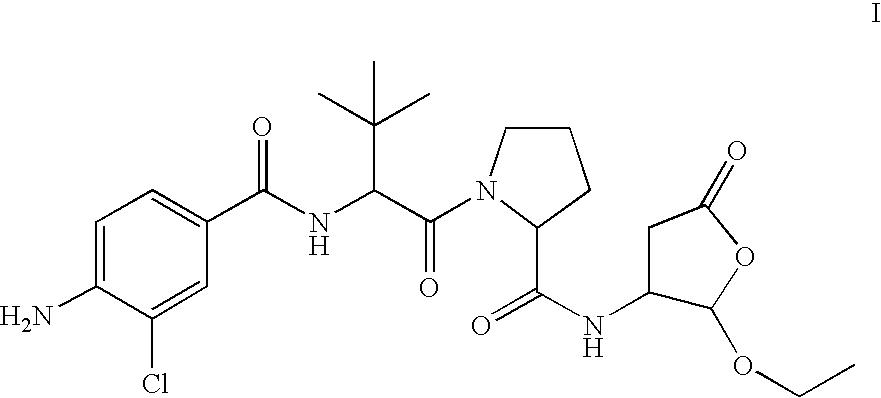
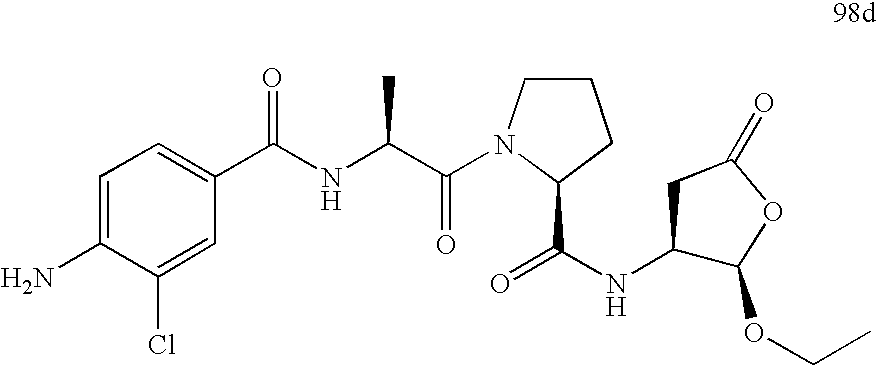
Prodrug of an ice inhibitor
This invention describes an ICE inhibitor prodrug (I) having good bioavailabilityCompound I is useful for treating IL-1 mediated diseases such as rheumatoid arthritis, inflammatory bowel disease, Crohn's disease, ulcerative colitis, inflammatory peritonitis, septic shock, pancreatitis, traumatic brain injury, organ transplant rejection, osteoarthritis, asthma, psoriasis, Alzheimer's disease, myocardial infarction, congestive heart failure, Huntington's disease, atherosclerosis, atopic dermatitis, leukemias and related disorders, myelodysplastic syndrome, uveitis or multiple myeloma.
Owner:VERTEX PHARMA INC
Azacitidine freeze-drying powder injection and preparation method thereof
InactiveCN101632643ANot prone to oxidationLong storage timePowder deliveryOrganic active ingredientsVitamin CMANNITOL/SORBITOL
The invention discloses a medicinal azacitidine freeze-drying powder injection for treating myelodysplastic syndrome and a preparation method thereof. The prescription of the azacitidine freeze-drying powder injection comprises azacitidine, mannitol and vitamin C. The invention solves the problem of rapid impurity increase caused by different crystal forms formed in the processes of rapidly freezing and drying the prior powder injection by optimizing the prescription and improving the preparation method.
Owner:HANGZHOU XIANDA MEDICINE TECH
Low Dose Therapy Of DNA Methylation Inhibitors
Methods are provided for treating patients with hematological disorders such as acute myeloid leukemia (AML), chronic myelogenous leukemia (CML), and the myelodysplastic syndromes (MDS). By administering a DNA methylation inhibitor to the patients following unique dosing regimens, the diseases can be efficaciously treated with reduced toxic side effects.
Owner:SUPERGEN
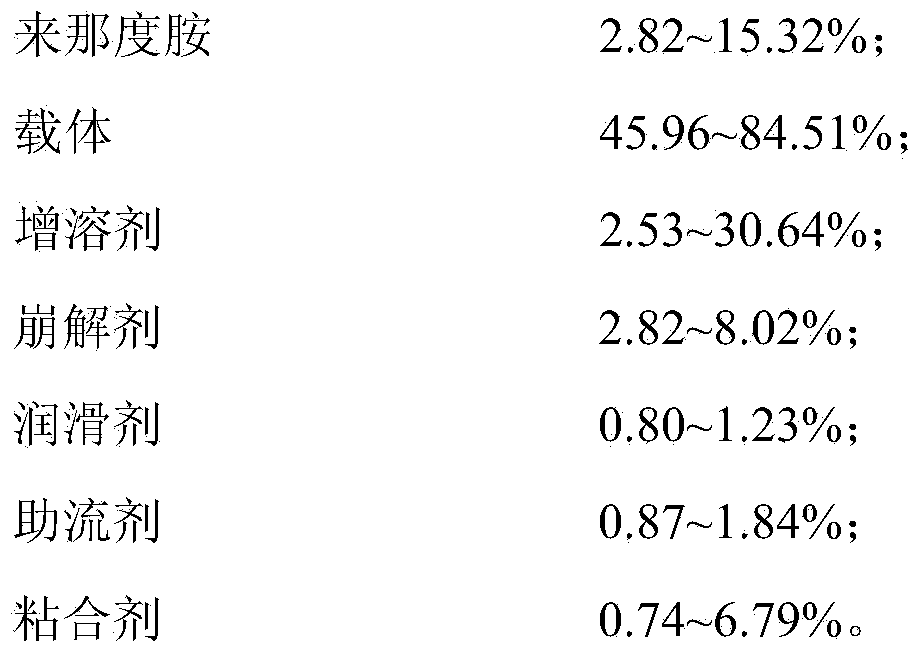
Composite for treating myelodysplastic syndrome and preparation method thereof
ActiveCN103705485ASimple granulation processControllable flyingOrganic active ingredientsPill deliverySolubilityAdhesive
The invention discloses a composite for treating myelodysplastic syndrome. The composite comprises the following components: lenalidomide, a carrier, a solubilizing agent, a disintegrating agent, a lubricating agent, a flow aid and an adhesive, wherein the carrier is a mixture of any one or several of a high-molecular water-soluble polymer, a water-soluble small molecule compound, a hydrophilic auxiliary material and an inorganic carrier; the solubilizing agent is a mixture of one or several of lauryl sodium sulfate, poloxamer, beta. cyclodextrin and a derivative thereof, polysorbate and polyoxyethylene alkyl ether. The invention also provides a preparation method of the composite, and the preparation method comprises the steps of grinding, mixing, dry granulation, total mixing and tabletting or capsule filling. The preparation method disclosed by the invention effectively enhances the water solubility and bioavailability of the lenalidomide; the dry granulation preparation process simplifies the preparation steps, reduces the cost, saves the energy resources, reduces the labor expenditure and realizes the energy conservation and environment protection in production.
Owner:AC PHARMA CO LTD
Method and system for analysis of flow cytometry data using support vector machines
ActiveUS8682810B2Digital computer detailsCharacter and pattern recognitionSupport vector machineAlgorithm
An automated method and system are provided for receiving an input of flow cytometry data and analyzing the data using one or more support vector machines to generate an output in which the flow cytometry data is classified into two or more categories. The one or more support vector machines utilize a kernel that captures distributional data within the input data. Such a distributional kernel is constructed by using a distance function (divergence) between two distributions. In the preferred embodiment, a kernel based upon the Bhattacharyya affinity is used. The distributional kernel is applied to classification of flow cytometry data obtained from patients suspected having myelodysplastic syndrome.
Owner:HEALTH DISCOVERY CORP
Methods for diagnosis of myelodysplastic syndromes (MDS)
ActiveUS20120028276A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSurface markerRegimen
The present invention relates to methods and kits for diagnosing, ascertaining the clinical course of myelodysplastic syndrome (MDS) and ascertaining response to a therapy regimen of myelodysplastic syndrome. Specifically the invention provides methods and kits useful in the diagnosis and determination of clinical parameters associated with MDS based on surface markers unique to MDS.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
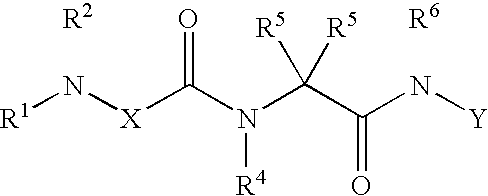
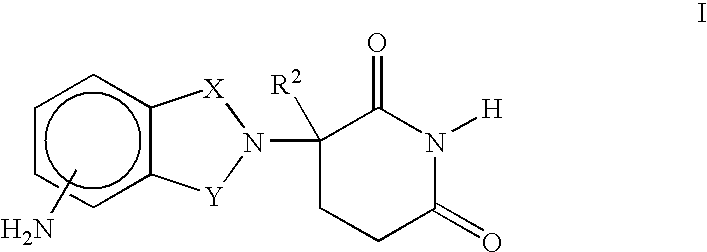
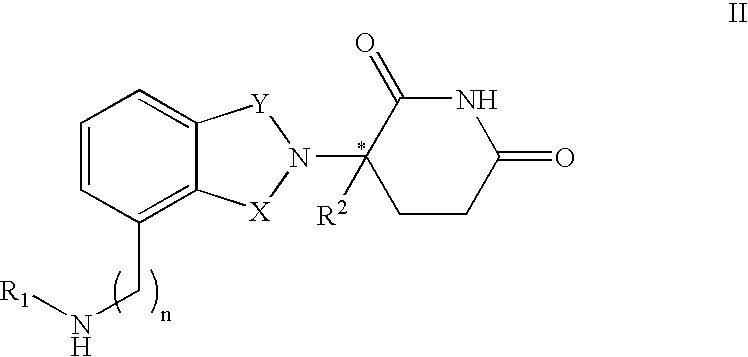
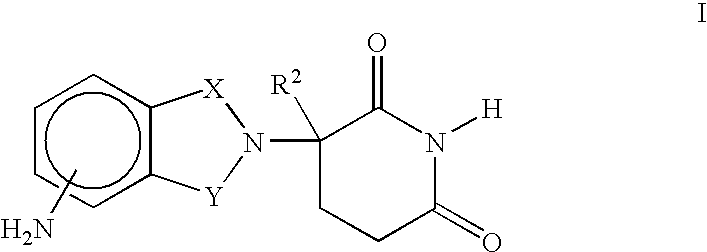
Composition and methods for the treatment of myelodysplastic syndrome and acute myeloid leukemia
Methods and compositions are provided for treating myelodysplastic syndrome and acute myeloid leukemia, wherein the composition comprises at least one compound according to Formula I:wherein R1 is selected from the group consisting of —NH2, —NH—CH2—CO2H, —NH—CH(CH3)—CO2H, and —NH—C(CH3)2—CO2H, or a pharmaceutically acceptable salt of such a compound; and a DNA methyltransferase inhibitor, or a pharmaceutically acceptable salt thereof.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
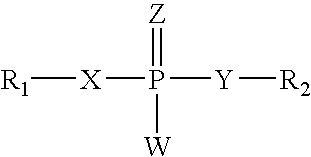
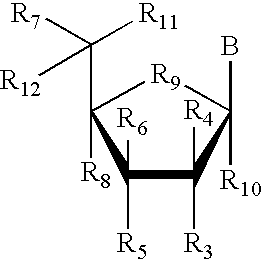
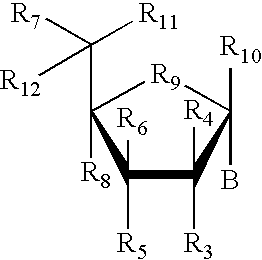






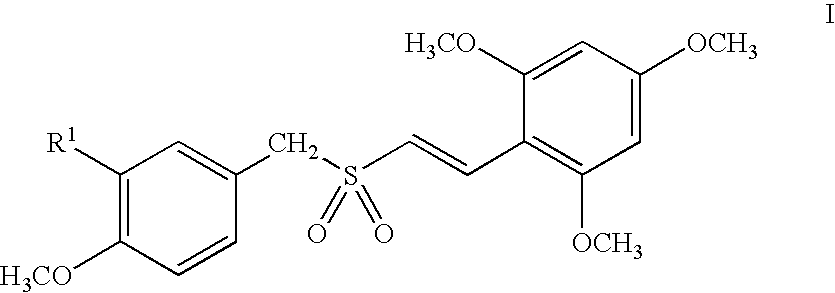
![Substituted pyrazolo[3,4-d]pyrimidines as PI3K inhibitors Substituted pyrazolo[3,4-d]pyrimidines as PI3K inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/afcc1086-f7bf-415b-a630-8356258d7962/US07919498-20110405-C00001.png)
![Substituted pyrazolo[3,4-d]pyrimidines as PI3K inhibitors Substituted pyrazolo[3,4-d]pyrimidines as PI3K inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/afcc1086-f7bf-415b-a630-8356258d7962/US07919498-20110405-C00002.png)
![Substituted pyrazolo[3,4-d]pyrimidines as PI3K inhibitors Substituted pyrazolo[3,4-d]pyrimidines as PI3K inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/afcc1086-f7bf-415b-a630-8356258d7962/US07919498-20110405-C00003.png)