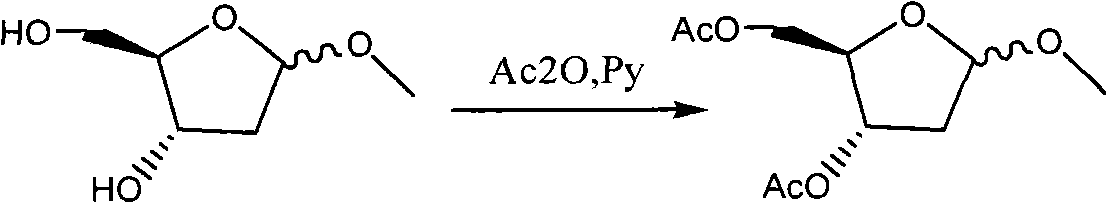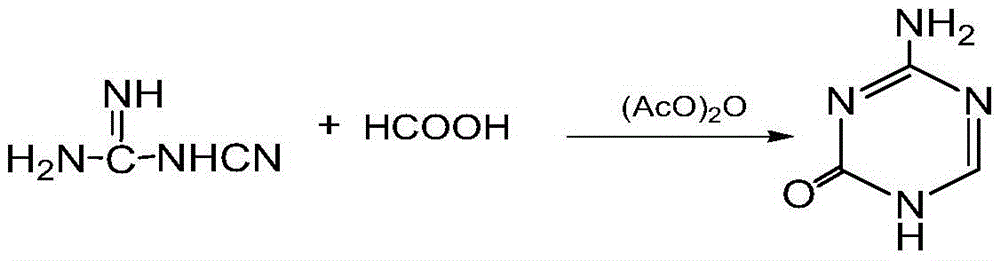Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "5-azacytosine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
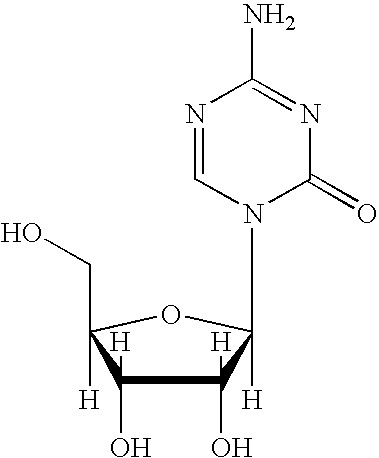
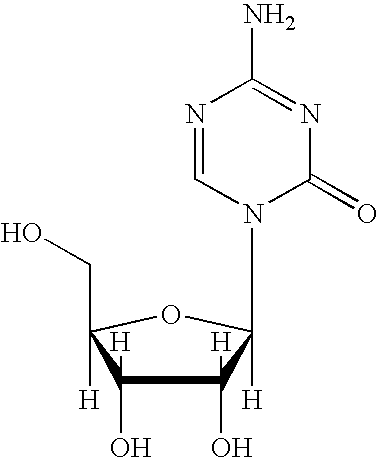
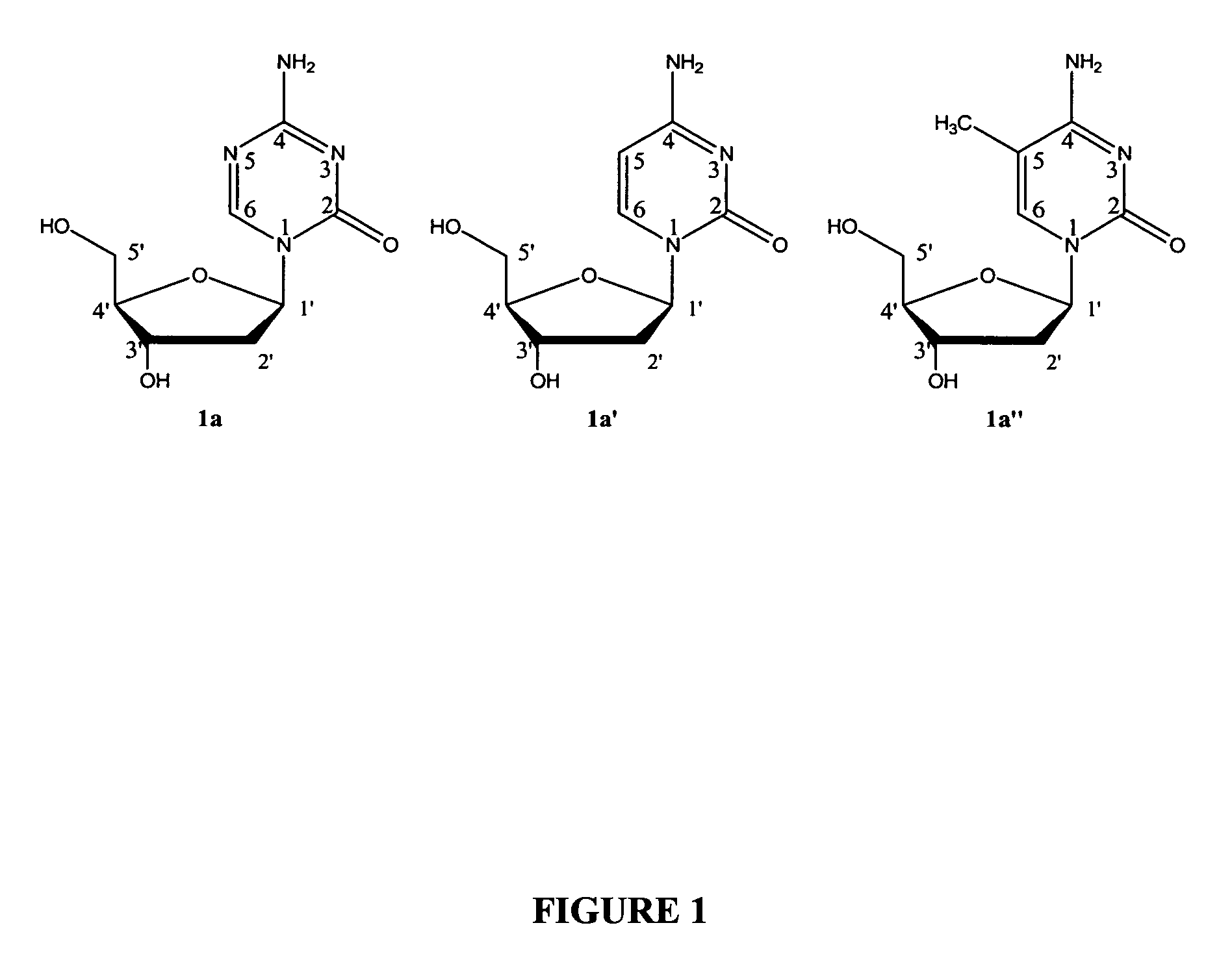
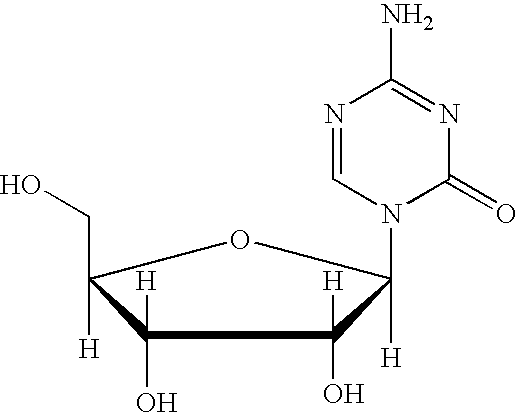
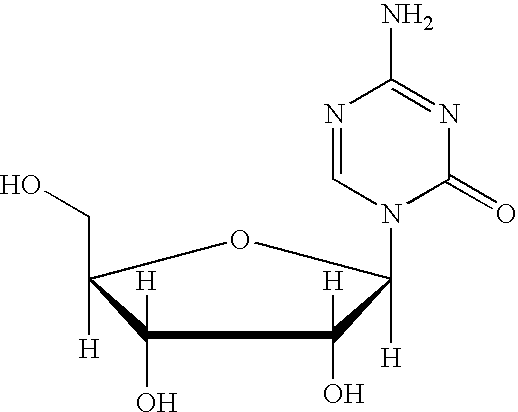
5-azacytosine is a monoamino-1,3,5-triazine that is cytosine in which the aromatic CH at position 5 is replaced by a nitrogen. Ontology Summary from ChEBI PubChem
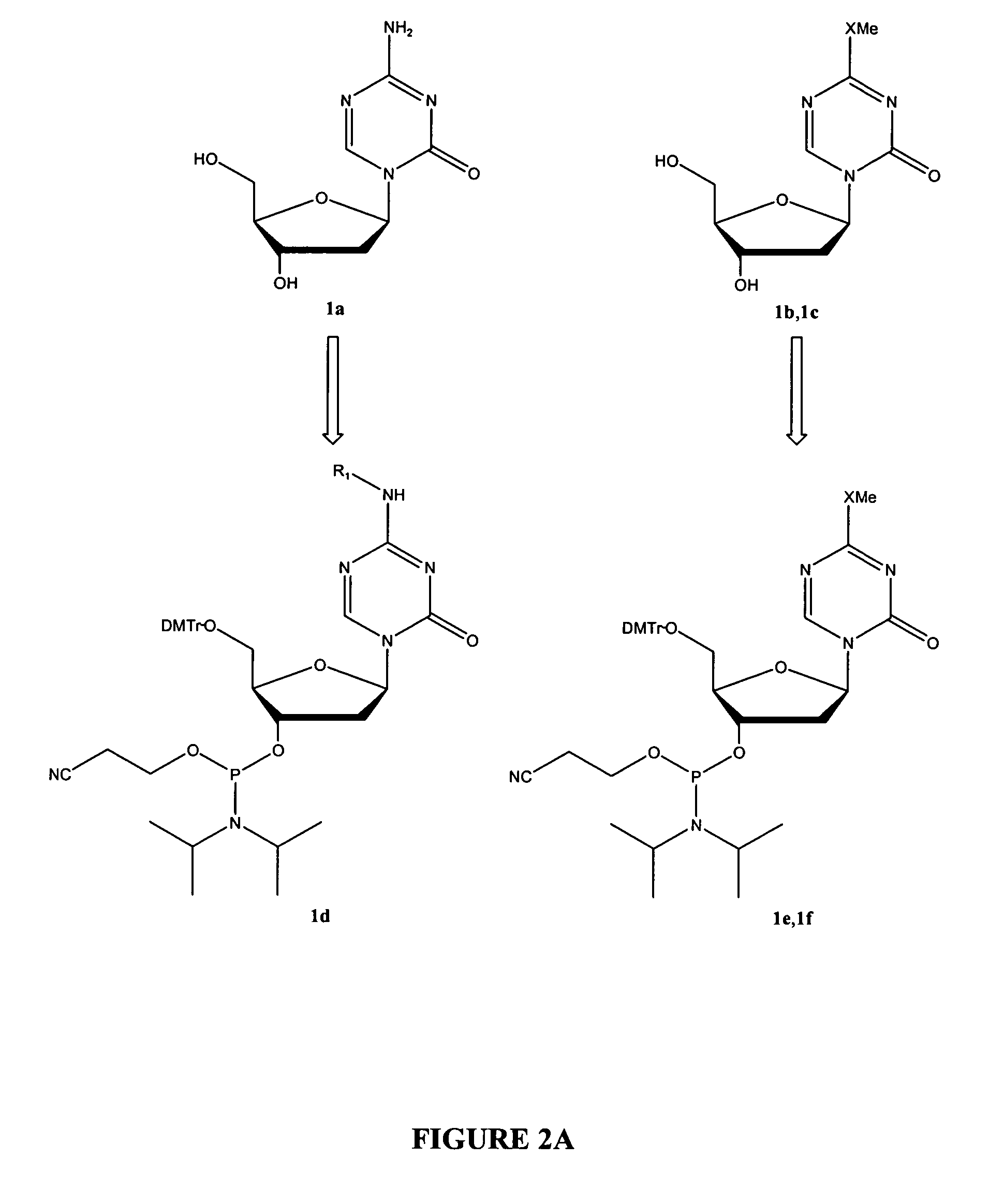
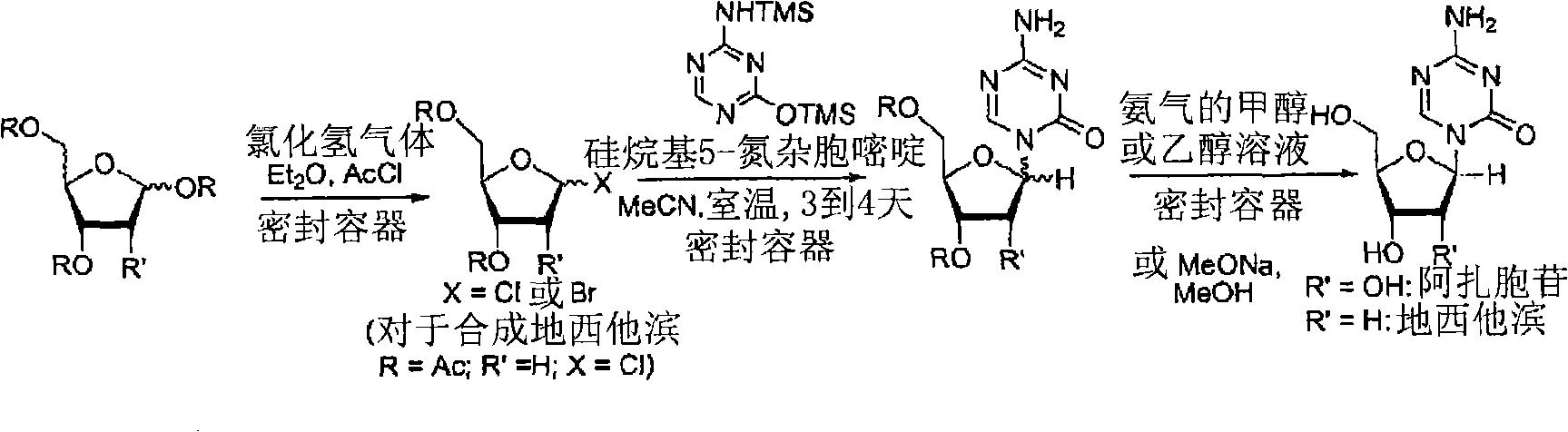
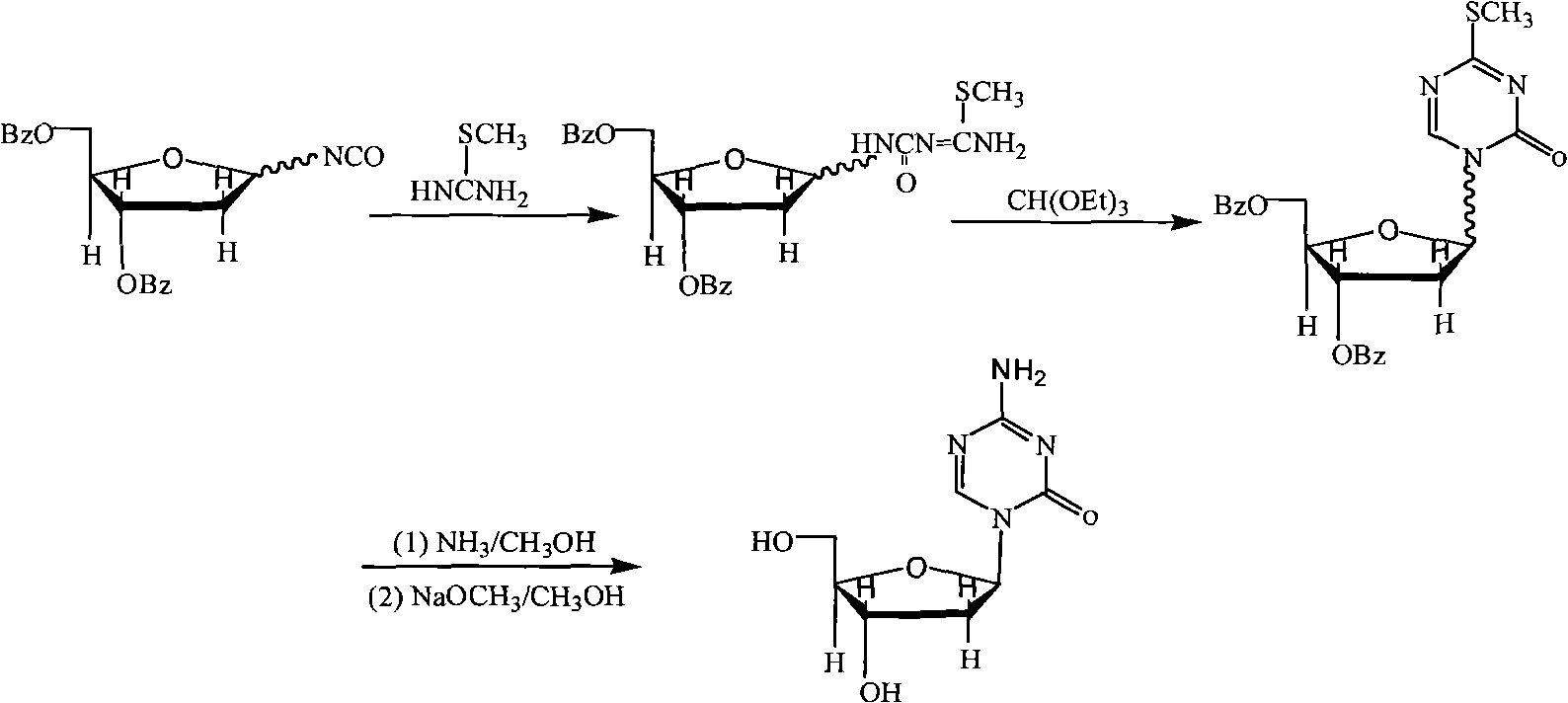
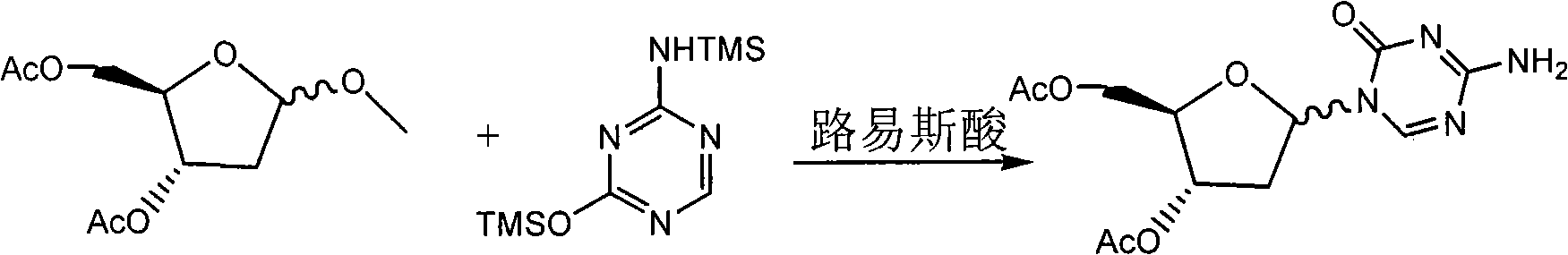
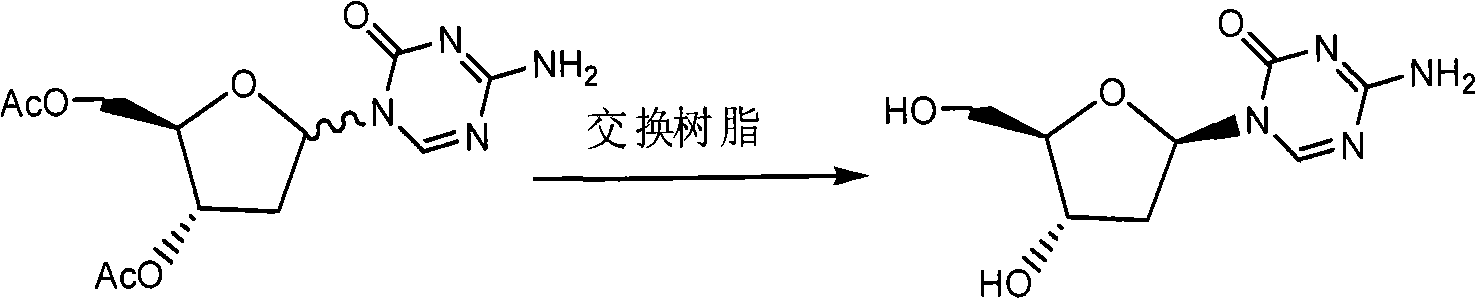
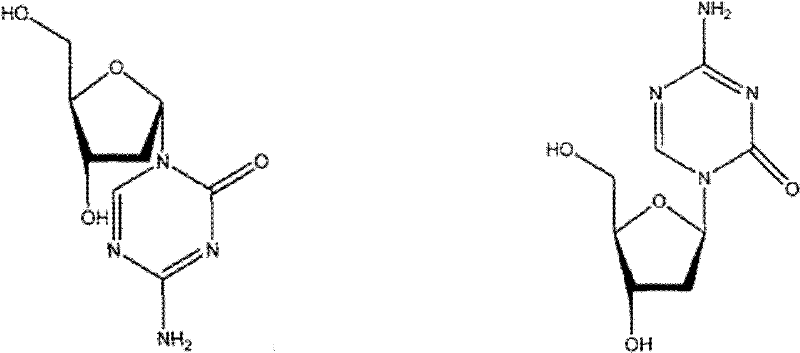
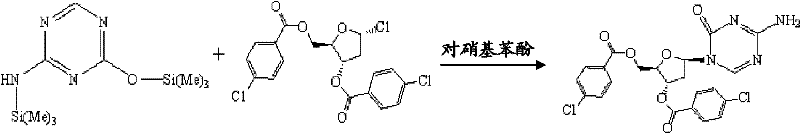
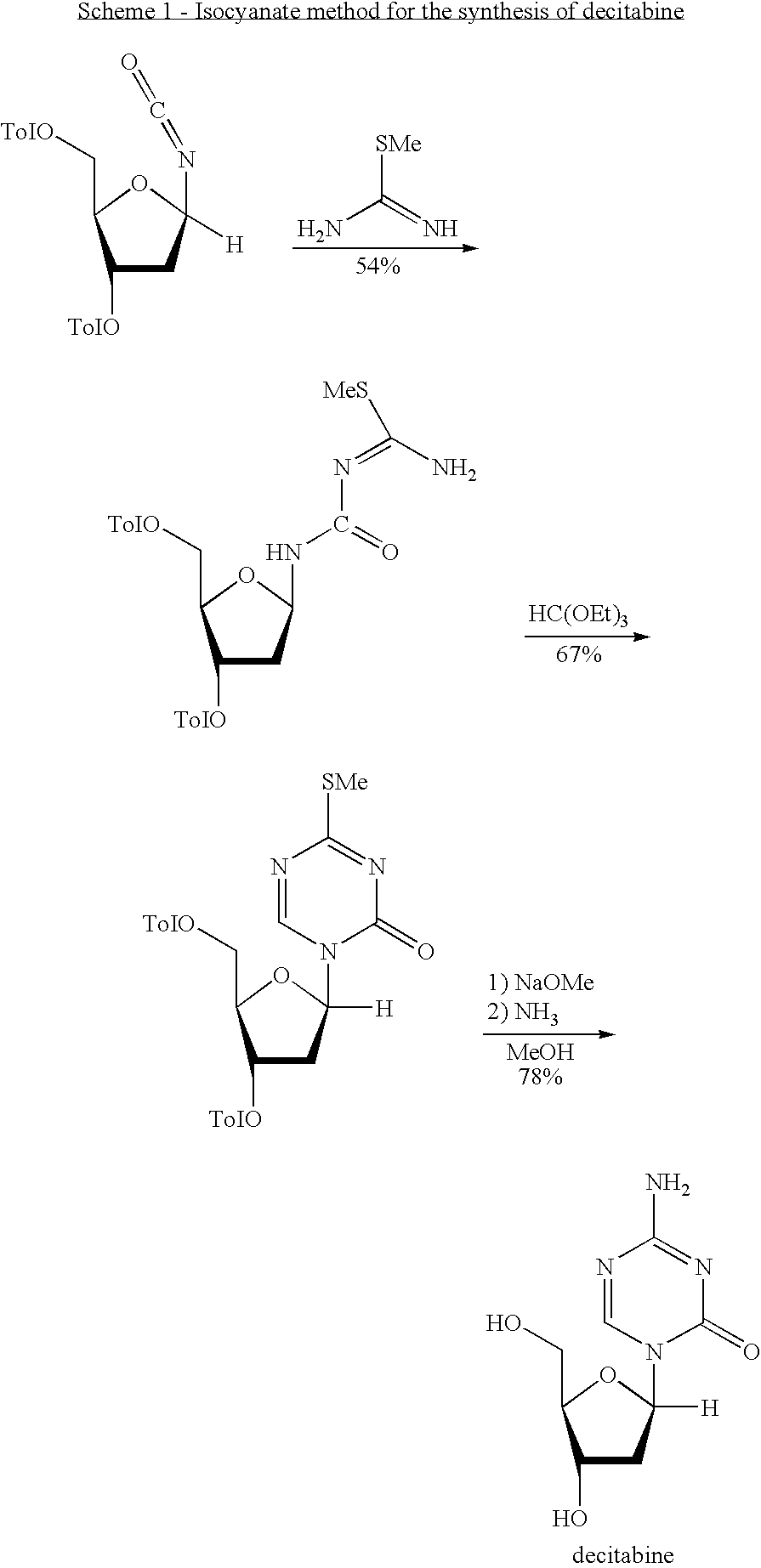
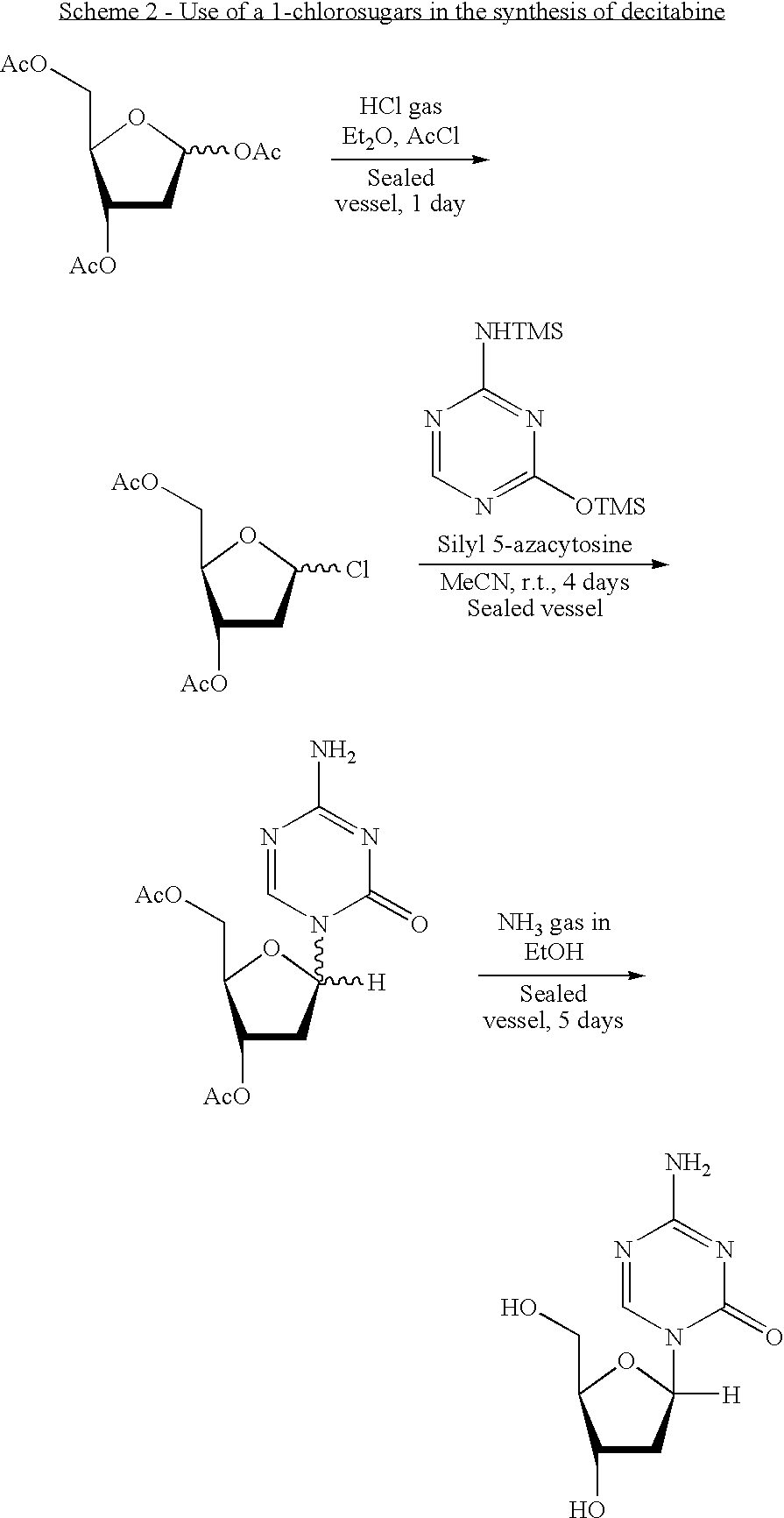
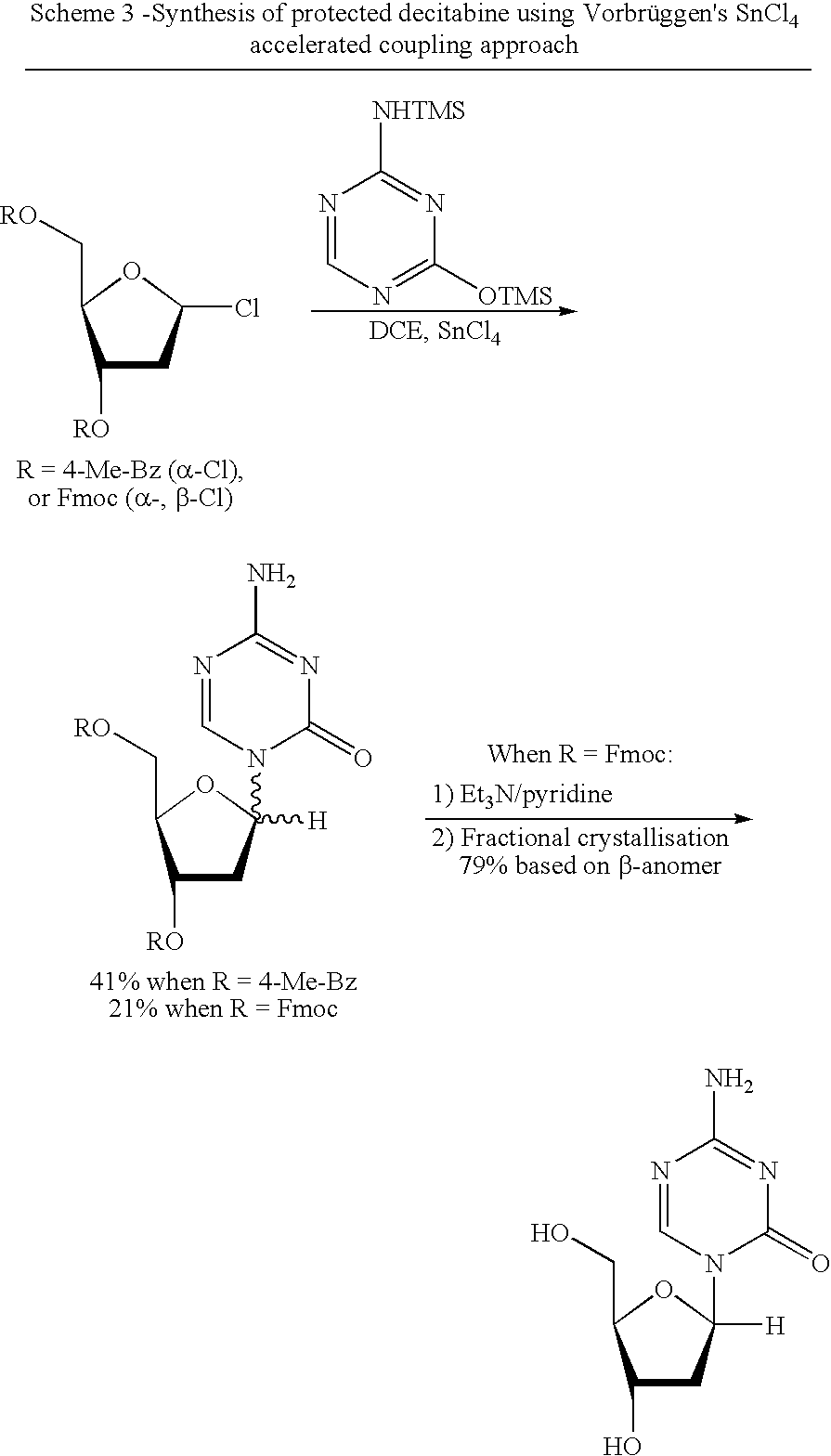
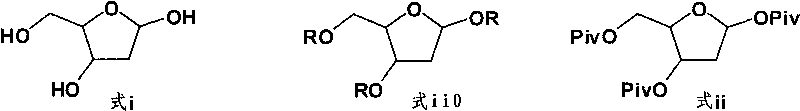
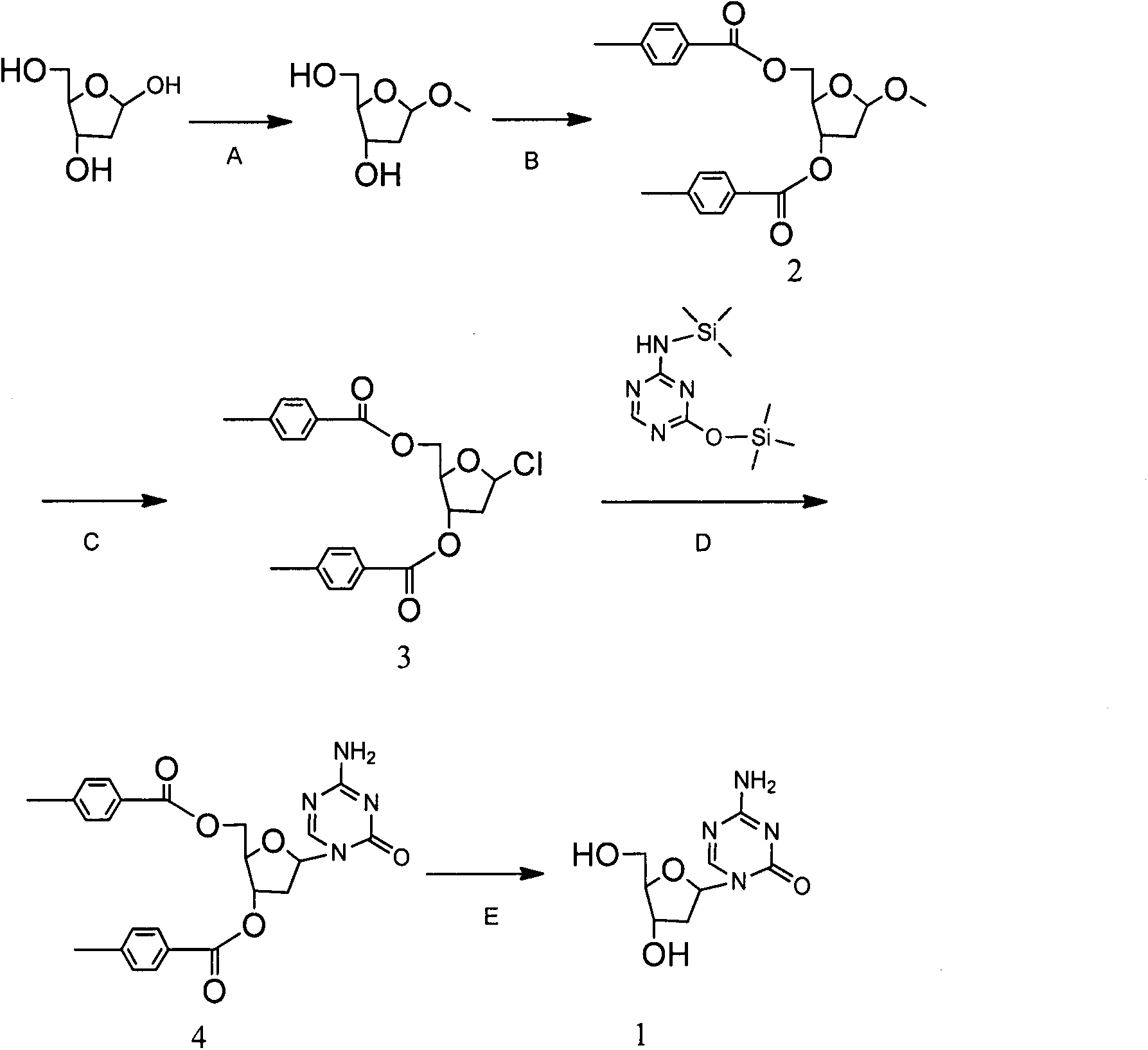
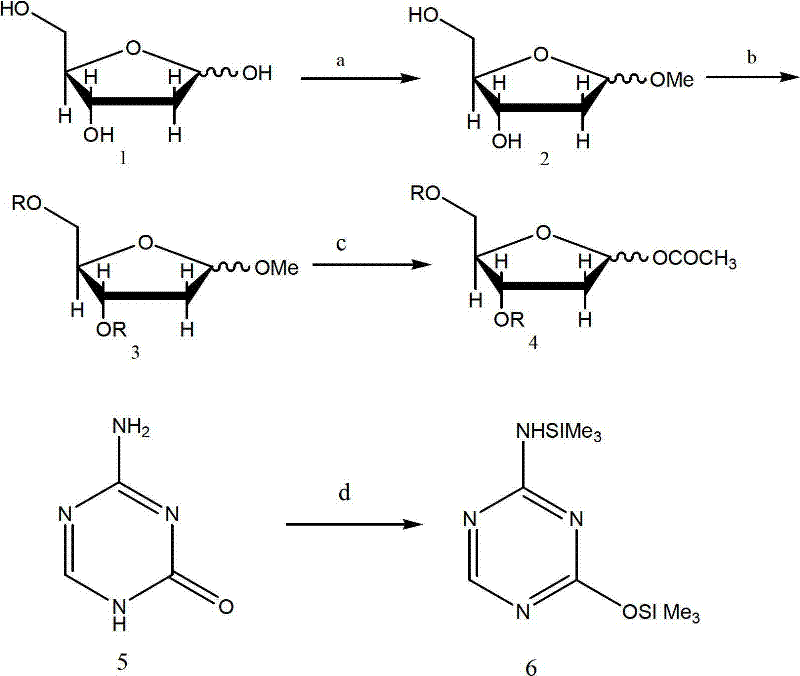
Synthesis of 5-azacytidine
InactiveUS7038038B2Amenable to scale-upAvoiding hydrolysis of the s-triazine ringBiocideSugar derivativesTrimethylsilyl trifluoromethanesulfonateCoupling
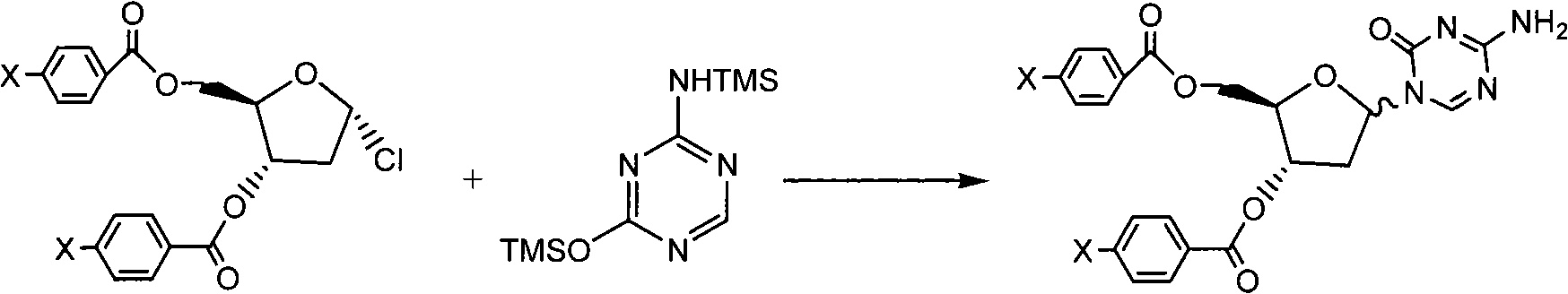
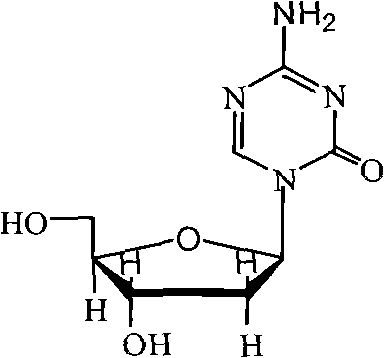
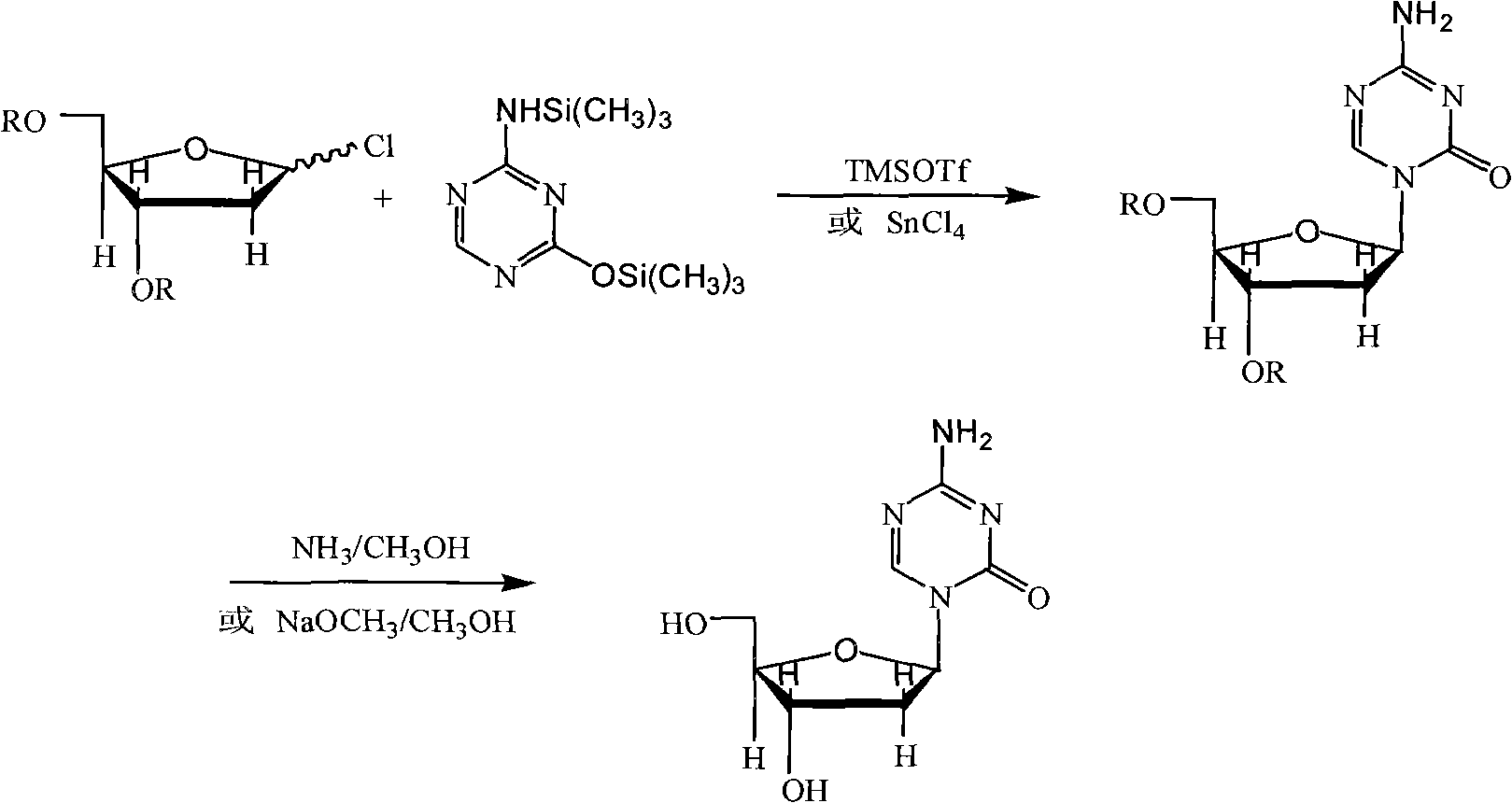
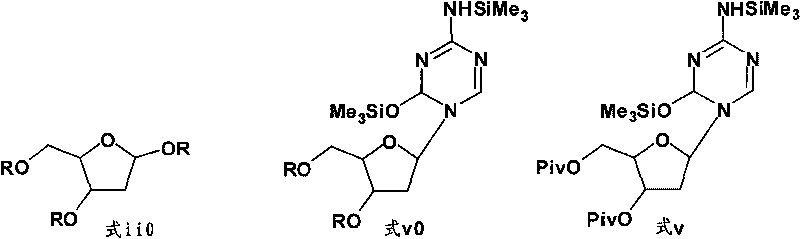
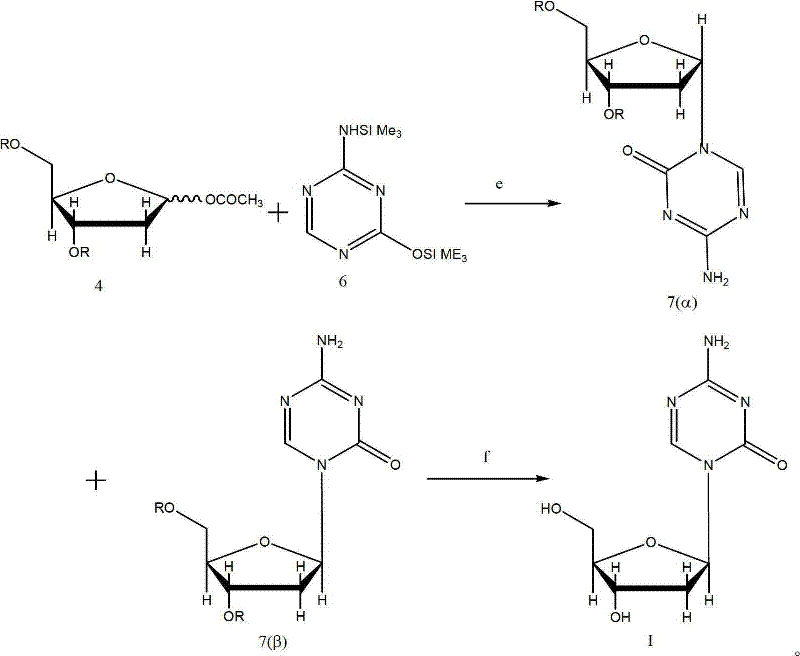
The present invention provides a method for the preparation of 5-azacytidine, wherein 5-azacytidine is represented by the structure: The method involves the silylation of 5-azacytosine, followed by the coupling of silylated 5-azacytosine to a protected β-D-ribofuranose derivative. The coupling reaction is catalyzed by trimethylsilyl trifluoromethanesulfonate (TMS-Triflate).
Owner:PHARMION
Oligonucleotide analogues incorporating 5-aza-cytosine therein
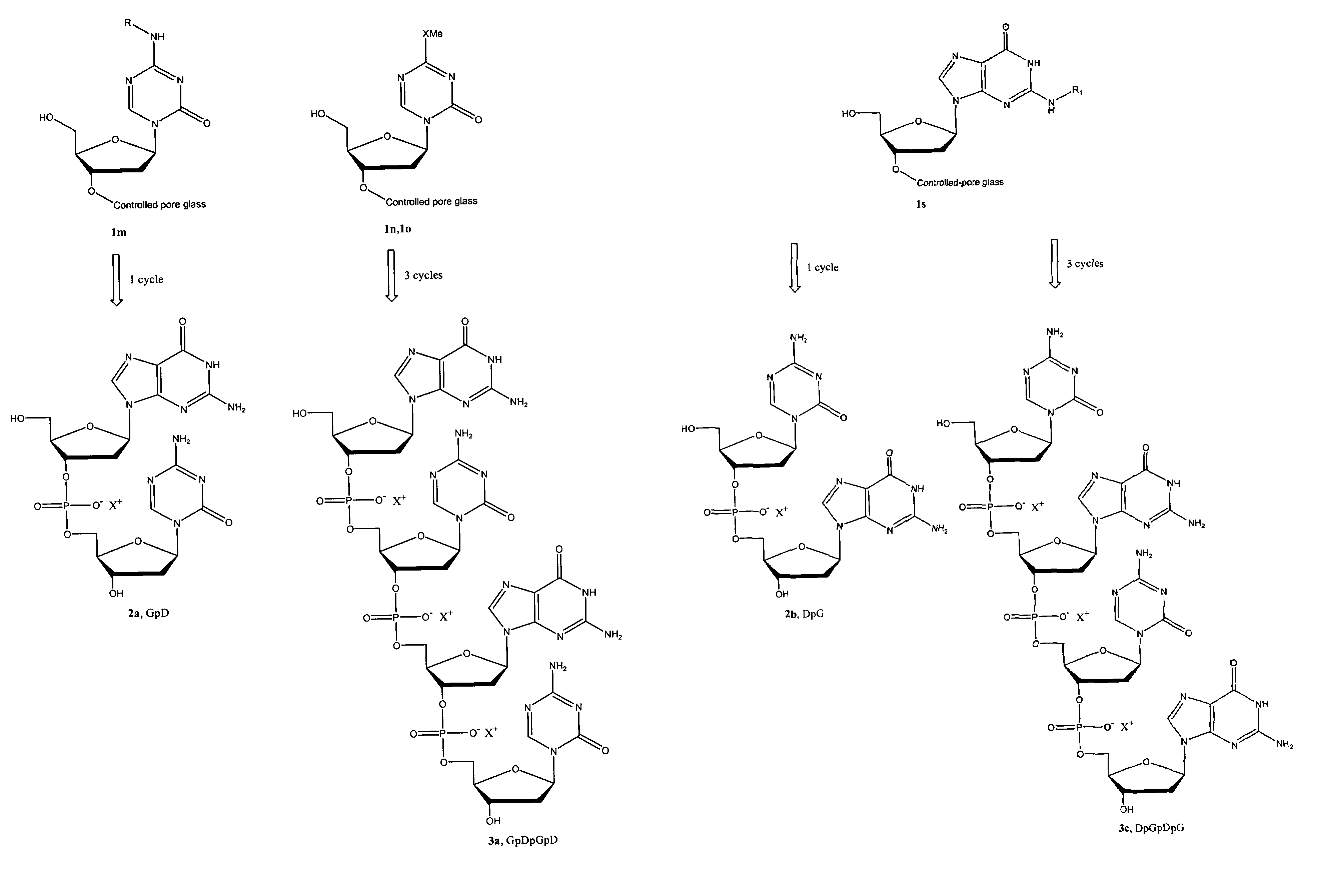
Oligonucleotide analogues are provided that incorporate 5-aza-cytosine in the oligonucleotide sequence, e.g., in the form of 5-aza-2′-deoxycytidine (decitabine) or 5-aza-cytidine. In particular, oligonucleotide analogues rich in decitabine-deoxyguanosine islets (DpG and GpD) are provided to target the CpG islets in the human genome, especially in the promoter regions of genes susceptible to aberrant hypermethylation. Such analogues can be used for modulation of DNA methylation, such as effective inhibition of methylation of cytosine at the C-5 position. Methods for synthesizing these oligonucleotide analogues and for modulating nucleic acid methylation are provided. Also provided are phosphoramidite building blocks for synthesizing the oligonucleotide analogues, methods for synthesizing, formulating and administering these compounds or compositions to treat conditions, such as cancer and hematological disorders.
Owner:SUPERGEN
Process for Making 5-Azacytosine Nucleosides and Their Derivatives
ActiveUS20100036112A1High boiling pointIncrease polarityEsterified saccharide compoundsOrganic active ingredientsDecitabineSilylation
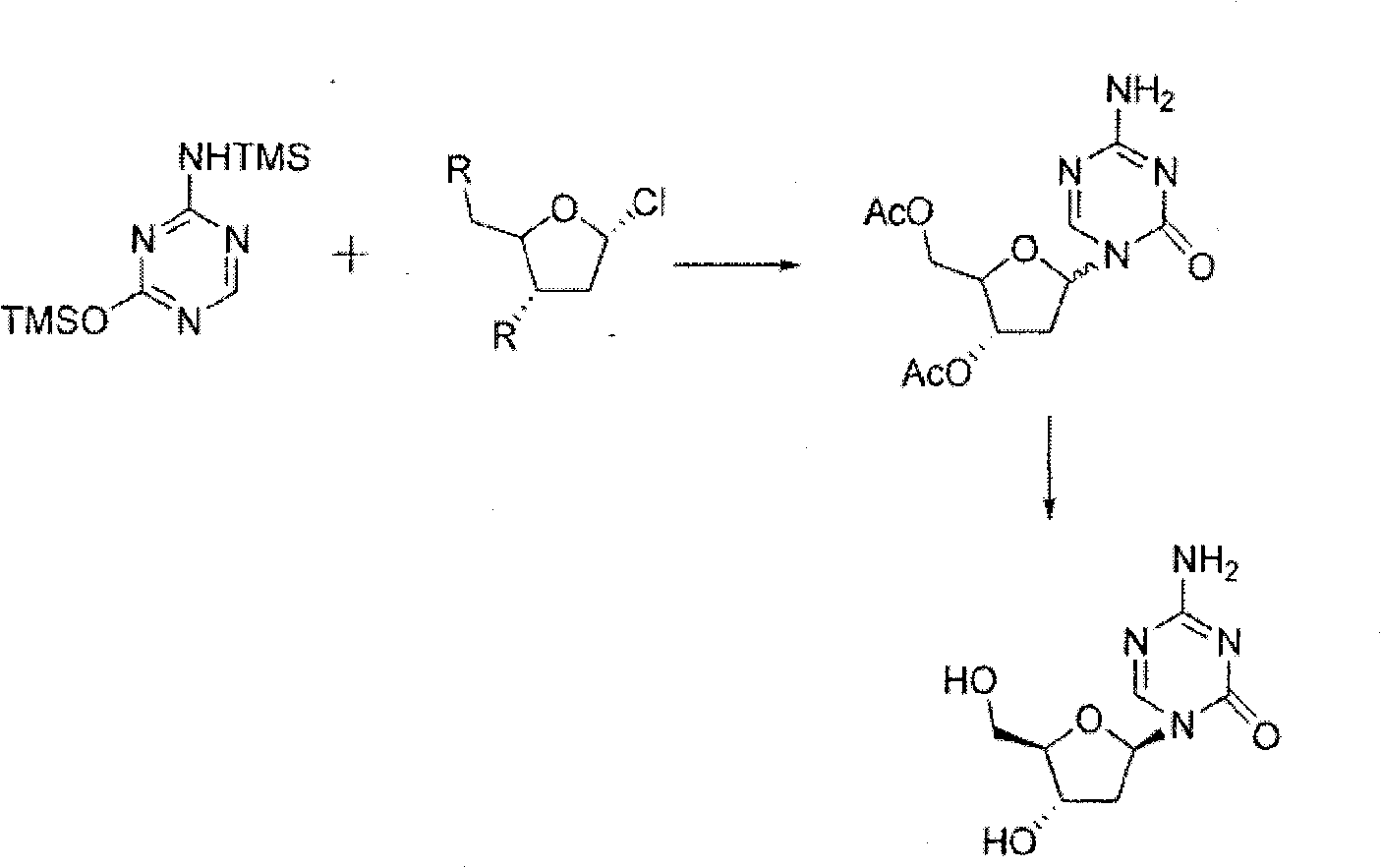
A process of synthesizing a 5-azacytosine nucleoside, such as azacitidine and decitabine, comprises coupling a silylated 5-azacytosine with a protected D-ribofuranose of formula in the presence of a sulfonic acid catalyst.
Owner:SCINOPHARM TAIWAN LTD
Synthesis of 5-Azacytidine
InactiveUS20060247432A1Amenable to scale-upAvoiding hydrolysis of the s-triazine ringSugar derivativesCarbohydrate active ingredientsTrimethylsilyl trifluoromethanesulfonateFuran
The present invention provides a method for the preparation of 5-azacytidine, wherein 5-azacytidine is represented by the structure: The method involves the silylation of 5-azacytosine, followed by the coupling of silylated 5-azacytosine to a protected β-D-ribofuranose derivative. The coupling reaction is catalyzed by trimethylsilyl trifluoromethanesulfonate (TMS-Triflate).
Owner:PHARMION
Process for making 5-azacytosine nucleosides and their derivatives
ActiveCN102216315AShorten the timeSuitable for large-scale synthesisEsterified saccharide compoundsBiocideDecitabine5-azacytosine
A process of synthesizing a 5-azacytosine nucleoside, such as azacitidine and decitabine, comprises coupling a silylated 5-azacytosine with a protected D-ribofuranose of formula in the presence of a sulfonic acid catalyst.
Owner:SCINOPHARM TAIWAN LTD
Decitabine synthesis and industrial production method
ActiveCN102827224AAvoid the problem of excessive heavy metalsControl volume and reaction conditionsSugar derivativesSugar derivatives preparationGlucosideMethanol
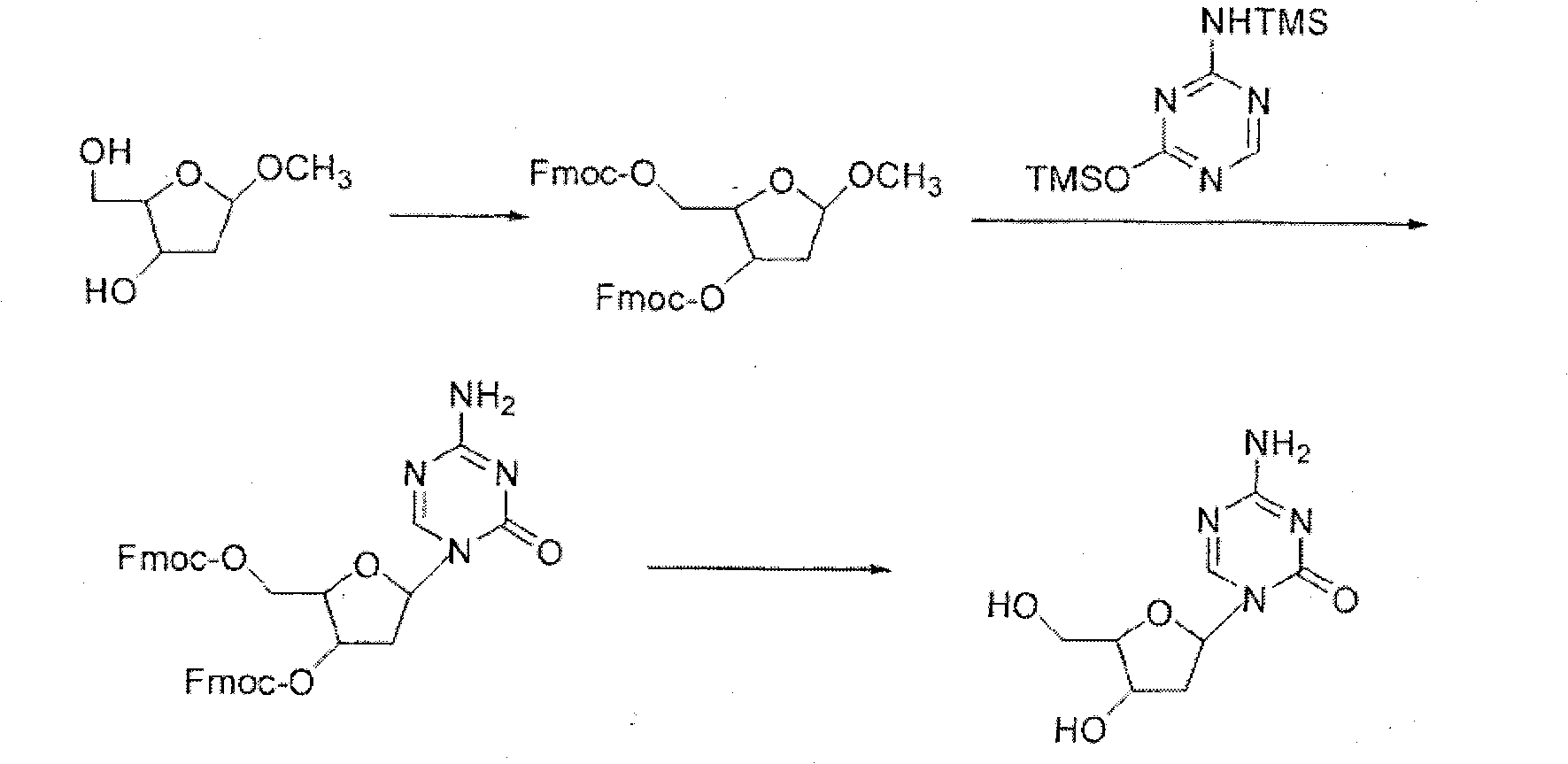
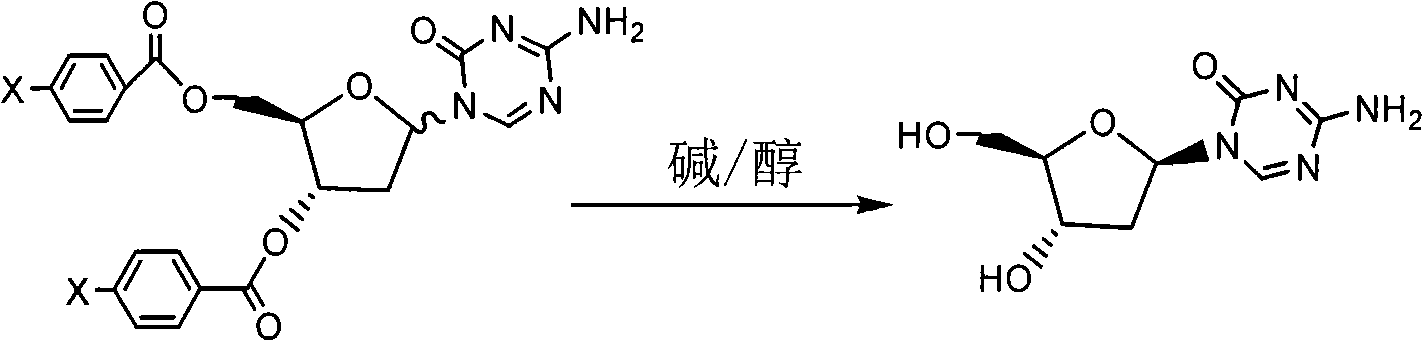
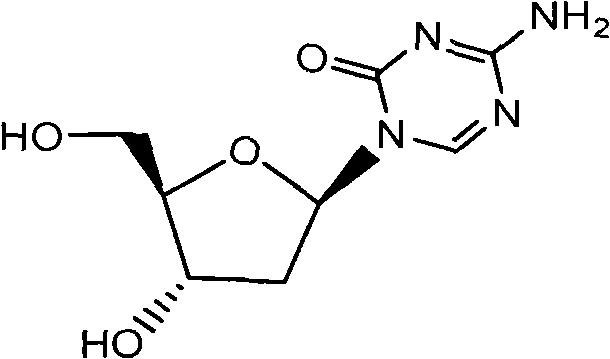
The invention relates to a decitabine synthesis and industrial production method, which comprises the following steps of: using 2-Deoxy-D-ribose as a raw material, performing a reaction between the raw material and methanol to obtain glucoside, protecting 3,5-dihydroxy by the use of 9-fluorenylmethyloxycarbonyl, reacting with hydrogen chloride to obtain 1-chlorflurecol sugar, performing a reaction between 1-chlorflurecol sugar and silanized 5-azacytosine, carrying out deprotection, and refining to obtain decitabine. The invention is characterized in that stannic chloride is not required during the condensation reaction between 1-chlorflurecol sugar and silanized 5-azacytosine so as to avoid the problem of excessive contents of heavy metals in pharmaceutical materials; and simultaneously the amount of trimethylsilyl trifluoromethanesulfonate and reaction conditions are controlled so as to increase the body burden of beta in the product.
Owner:JIANGSU HANSOH PHARMA CO LTD
Method for preparing improved decitabine
InactiveCN101560232AHigh purityShort stepsOrganic active ingredientsSugar derivativesRiboseDecitabine
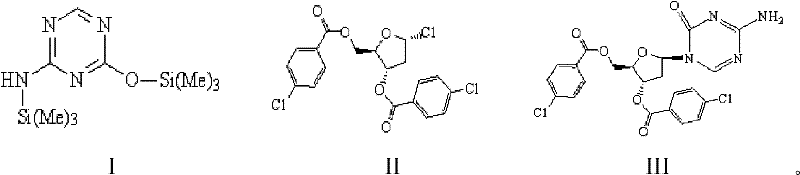
The invention discloses a method for preparing improved decitabine (formula I), comprising: using 1-alpha-chlorine-3,5-two pairs of halo-benzoyl-2-deoxidation-D-ribose (formula II) as materials, concentrating with 2-4-bi-(trimethyl silicane)-5-azacytosine (formula III) to obtain I by de-protecting group. Materials used in the invention are easy to obtain at a low price. The invention also has the advantages of convenient operation and a high reaction yield, and is suitable for industrialized production.
Owner:SHANGHAI QINGSONG PHARMA
Industrialized production method for high-purity decitabine
InactiveCN101948493AReduce the impactMeet quality requirementsSugar derivativesSugar derivatives preparationSodium methoxideSolvent
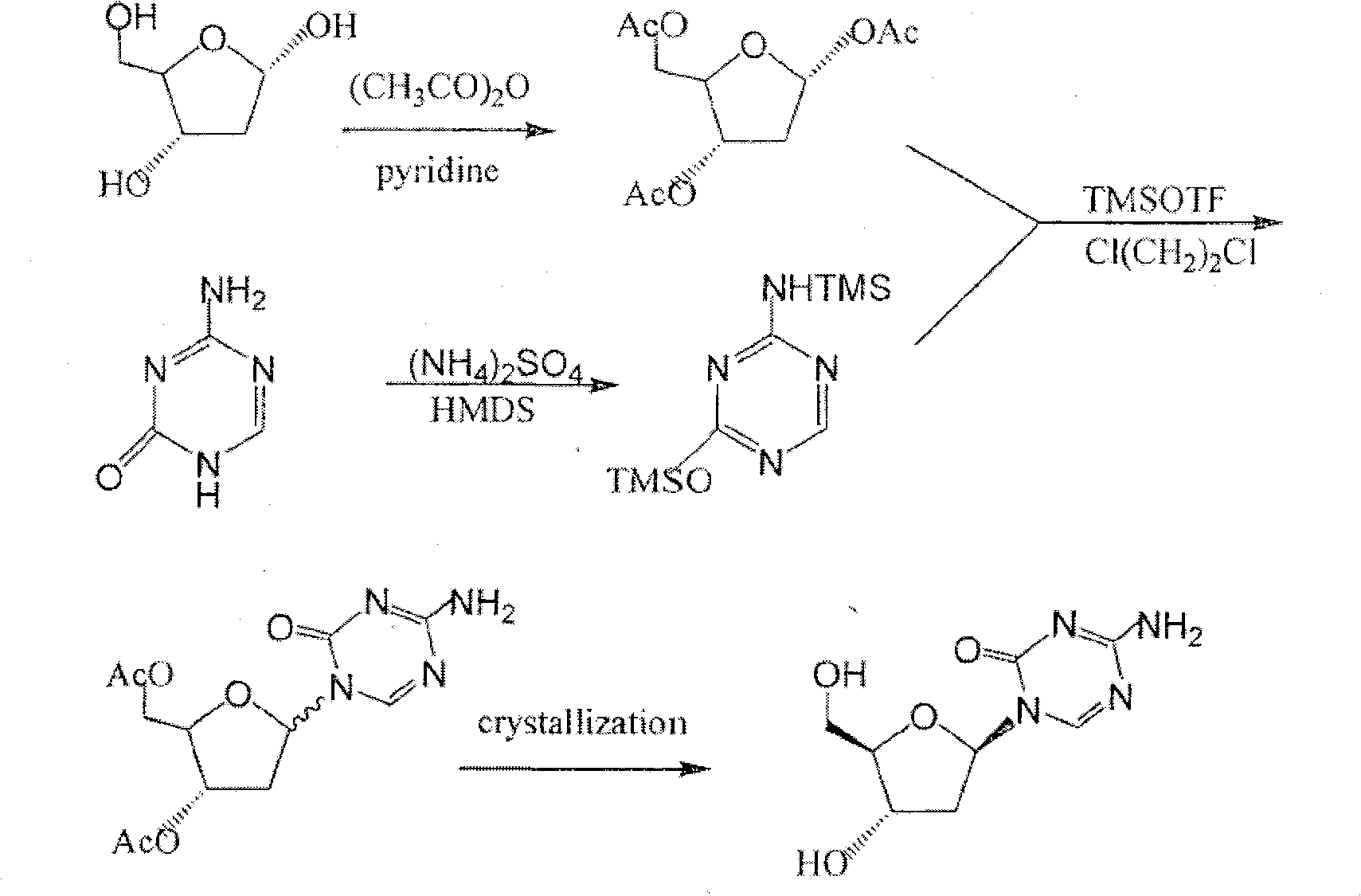
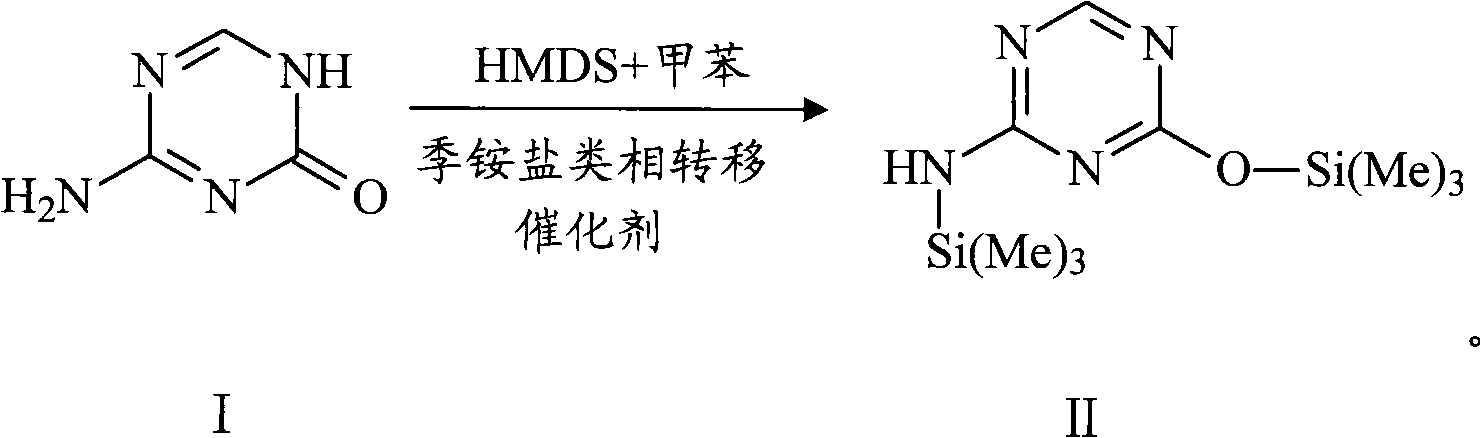
The invention provides an industrialized production method for high-purity decitabine. The method comprises the following steps of: 1, performing silanization reaction of 5-azacytosine, bis(trimethylsilyl)amine and trimethyl chlorosilane, which serve as raw materials, to prepare 2,4-bis(trimethylsilyl)-5-azacytosine; 2, performing reaction of the product obtained by the step 1 and 1-chloro-3,5-bis-(4-chlorobenzoyl)-2-deoxy-D-ribofuranose, which serve as raw materials, to prepare a crude product of 1-(3,5-bis-(4-chlorobenzoyl)-2-deoxy-beta-D-ribofuranose)-5-azacytosine; 3, dissolving the product obtained by the step 2 in a C5 to C7 hydrocarbon, stirring, filtering and drying to obtain a refined product; and 4, producing the high-purity decitabine by using the product obtained by the step 3, methyl alcohol and sodium methoxide as raw materials. The method overcomes the disadvantages of need of column purification, low purity, difficult industrial production in the prior art, and has the advantages of simple and convenient operation, small solvent consumption, small influence on the environment, low labor intensity, short period, high product purity, single impurity and less than 0.1 percent total impurity content.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Method for synthesizing azacitidine
ActiveCN101974051AShorten reaction timeReduce waste volumeSugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsAmmonium compoundsSynthesis methods
The invention relates to the field of pharmaceutical synthesis process and discloses a method for synthesizing azacitidine. The method comprises the following steps: performing silanization reaction on bis(trimethyl disilylamine) and 5-azacytosine by taking toluene as solvent under the catalysis of phase transfer catalyst of quaternary ammonium compounds under a moisture-isolated condition to generate a compound with structure of formula II; performing condensation reaction between the compound and ribose tetracetate to generate a compound with a structure of formula IV; and performing alcoholysis on the compound in an alkaline environment to generate the azacitidine. The synthesis method of the invention shortens the time of silanization reaction, reduces the waste amount of the bis(trimethyl disilylamine) and avoids influence of the moisture in methanol and ethanol in the purification stage on the purity of the finished product of azacitidine; moreover, the synthesis method has the advantages of cheap reagents, few reaction steps and mild reaction conditions and is favorable for industrial production.
Owner:重庆兴泰濠制药有限公司
Preparation method of decitabine
ActiveCN101560233AShort reaction stepsLow costSugar derivativesSugar derivatives preparationAcetic anhydrideIon-exchange resin
Owner:SHANGHAI QINGSONG PHARMA
Process for making 5-azacytosine nucleosides and their derivatives
ActiveUS8212021B2High boiling pointIncrease polarityEsterified saccharide compoundsOrganic active ingredientsSilylation5-azacytosine
Owner:SCINOPHARM TAIWAN LTD
Synthetic method of decitabine
ActiveCN102212097AIncrease contentReduce the difficulty of purificationSugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsCytimidineEthyl acetate
The invention relates to the field of medicament synthesis and discloses a synthetic method of decitabine. The method comprises the following specific steps of: undergoing a condensation reaction on 2,4-di-(trimethyl silicane)-5-aza-cytimidine and chloro-ribose under the catalytic action of nitrophenol; and hydrolyzing in an alkaline environment for removing acetyl to generate decitabine. In the synthetic method of decitabine, disclosed by the invention, nitrophenol is taken as a catalyst, the ratio of a beta configurational isomer in the obtained chloro-ribose aza-cytimidine is large, the content of beta configurational decitabine generated after hydrolysis is high, the purifying difficulty of decitabine is lowered, and the purity of a decitabine finished product is increased. During purification, ethyl acetate is used instead of methanol or ethanol, so that the influence of methanol or ethanol on the purity of the decitabine finished product at the purifying stage is avoided; and moreover, the synthetic method has the advantages of cheap reagents, less reaction steps, mild reaction condition and contribution to industrial production.
Owner:CHONGQING SINTAHO PHARM CO LTD
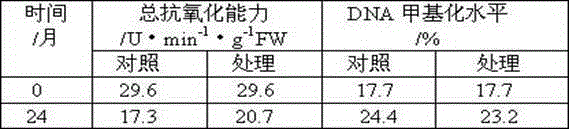
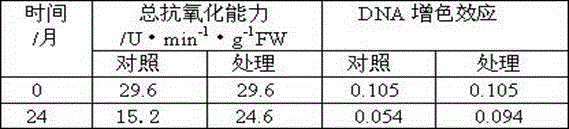
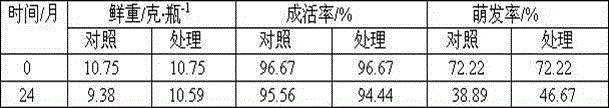
Long-term-subculture butterfly orchid protocrom-like body aging alleviating method
ActiveCN105325292ANo side effectsReduce proliferationPlant tissue cultureHorticulture methodsBetaineSide effect
A long-term-subculture butterfly orchid protocrom-like body aging alleviating method is as follows: an antioxidant, a DNA methylation inhibitor and a DNA structure stabilizer are added into a subculture medium for alleviating protocrom-like body aging and maintaining genetic material DNA stable, and the three types of regulators can be mixed simultaneously, can also be pairwise combined for use. The antioxidant mainly comprises ascorbic acid, cysteine salt, glutathione, sodium bisulfite, sodium selenite and thiourea. The DNA methylation inhibitor mainly comprises 5-aza-cytidine, betaine, choline, tea polyphenols and mannitol. The DNA structure stabilizer mainly comprises spermine, spermidine and putrescine. The long-term-subculture butterfly orchid protocrom-like body aging alleviating method can slow down long-term-subculture butterfly orchid protocrom-like body proliferation and decrease differentiation capacity, has certain repair effects on certain physiological and molecular markers of protocrom-like body, and is in no need of use of special equipment and easy to operate. The used chemical regulators have no toxic side effects on plant materials.
Owner:SUBTROPICAL CROPS INST OF FUJIAN PROVINCE
Synthesis of Decitabine
ActiveUS20100087637A1Increase productionSmall amountEsterified saccharide compoundsSugar derivativesCombinatorial chemistryDecitabine
A method for producing a β-enriched protected decitabine comprising:a) coupling a protected 2-deoxy-ribofuranose with a protected 5-azacytosine in the presence of a catalyst to form a reaction mixture comprising the protected decitabine of formula I; and b) quenching the reaction mixture of step a) with a base. The β-enriched protected decitabine so made may be deprotected to produce a decitabine product in a high yield and purity.
Owner:SCINOPHARM TAIWAN LTD
Method for preparing decitabine
InactiveCN101712708ARaw materials are easy to getThe total yield of the preparation process is highSugar derivativesAntineoplastic agentsTrimethylsilyl trifluoromethanesulfonateDecitabine
The invention discloses a method for preparing decitabine. The method comprises the following steps of: using 2-deoxy-D-ribose as a raw material; protecting the raw material by a pivaloyl group, and then reacting the raw material with alkylated 5-azacytosine under the action of a proper amount of a catalyst namely trimethylsilyl trifluoromethanesulfonate; performing hydrolysis to prepare a crude product of the decitabine without purification by a chromatography column; and performing recrystallization to prepare pure decitabine under the condition of a proper solvent. The method for preparing the decitabine greatly improves the total yield, greatly reduces the product cost, and is more suitable for large-scale industrial production.
Owner:SHANGHAI CHENPON PHARM TECH CO LTD
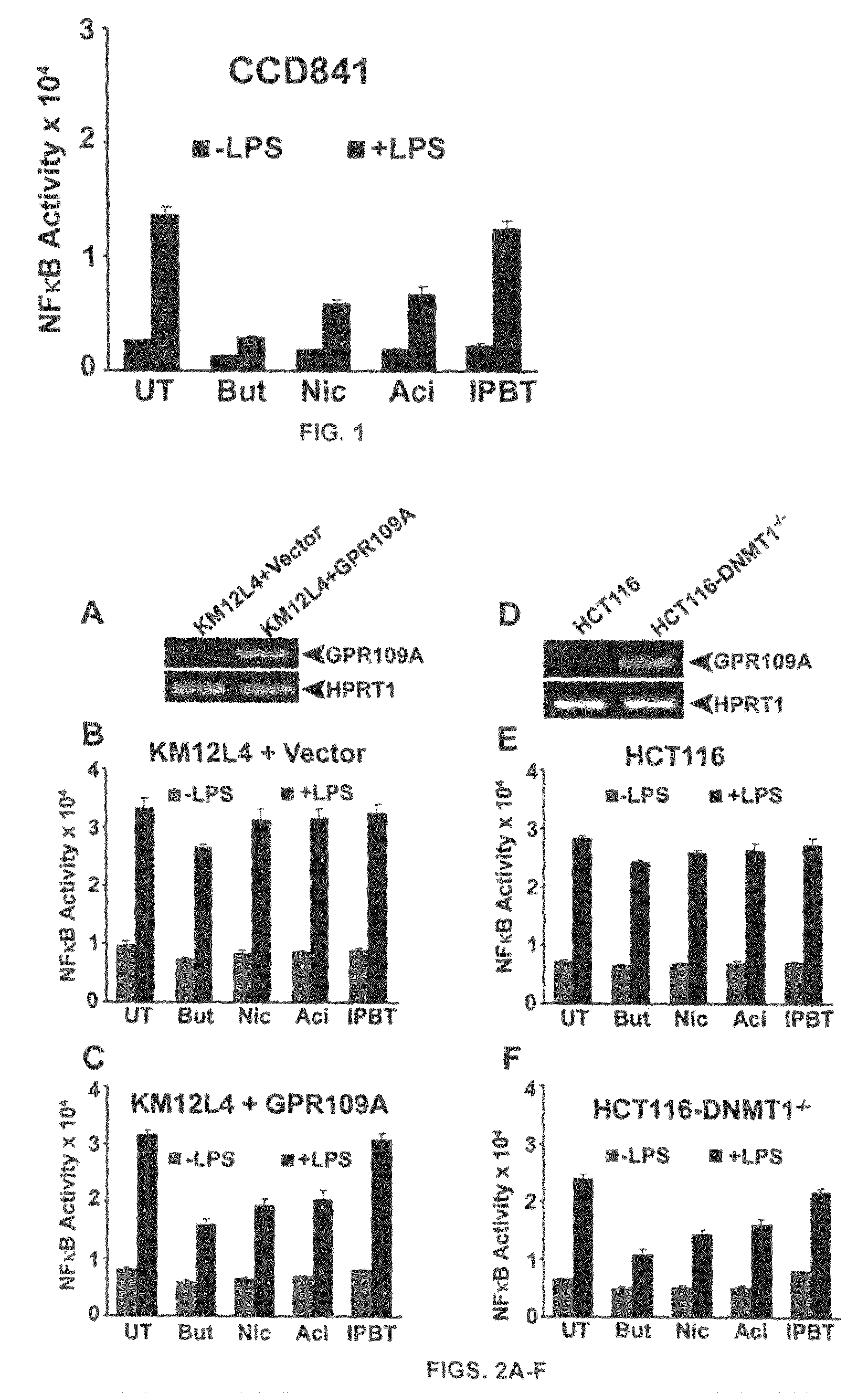
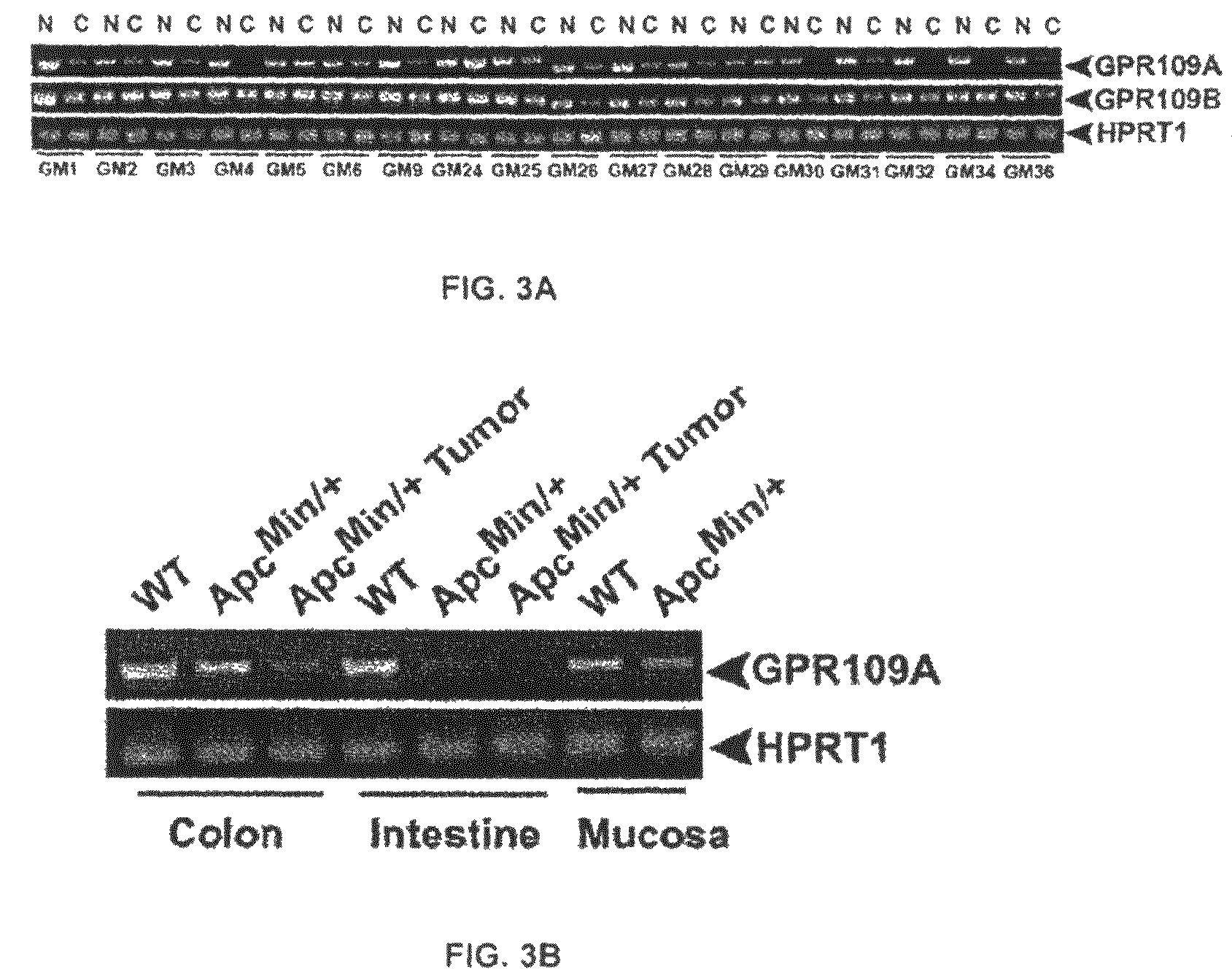
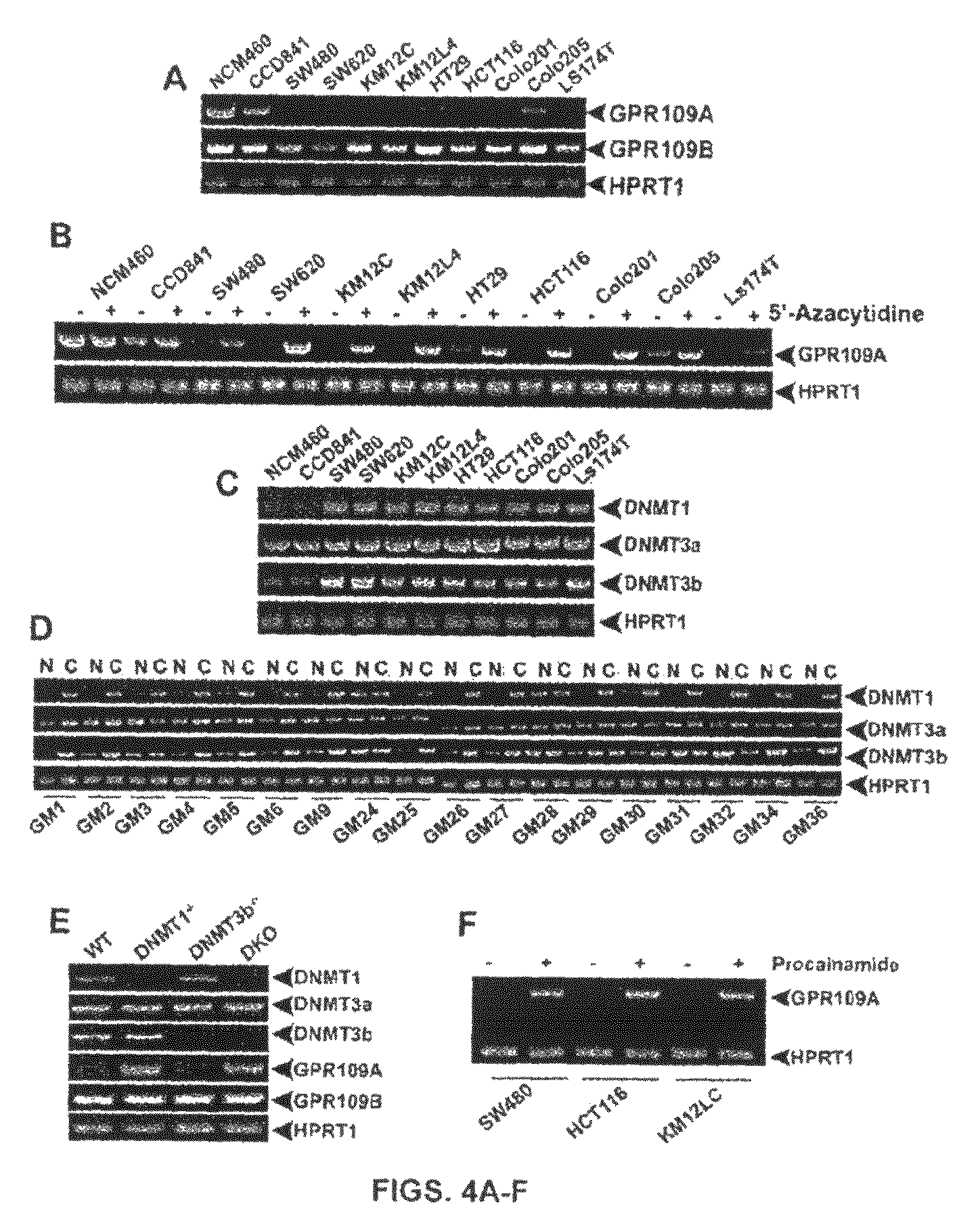
Compositions Comprising a GPR109 Ligand For Treating Disorders of the Digestive Tract and/or Cancer
Pharmaceutical compositions containing an effective amount of a ligand for GPR109 to decrease intracellular cAMP levels of a subject in combination with an effective amount of a DNA methyl transferase inhibito to reduce or inhibit downregulation of GPR109 in the intestinal epithelial cells of the subject relative to a control are provided. It has been discovered that ligands for GPR109 can be used to treat one or more symptoms of cancer, inflammatory disorders, and diarrhea. Representative CPR 109 ligands include, but are not limited to butyrate, β-hydroxybutyrate, nicotinic acid, acifran, and octanoate. Suitable DNA methyl transferase inhibitors include 5-azacytidine, 5-aza-2′-deoxytidine, 1-β-D-arabinfαmosyl-5-azacytosine and dihydro-5-azacytidine. Typically, the compositions are formulated to achieve a GPR 109 ligand serum blood level of about 1 to about 1000 μM. The compositions are useful for the treatment of one or more symptoms of cancer. Preferred cancers that can be treated using the disclosed compositions include, but are not limited to colon cancer, breast cancer and leukemia. Methods for treating cancer, inflammatory disorders, and diarrhea are also provided.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Carbon nitride and preparation method thereof
The invention discloses a preparation method of carbon nitride, which comprises the following step: carrying out high-temperature annealing on 5-azacytosine in a non-oxidizing atmosphere to prepare the carbon nitride, wherein the heating rate is 0.2-10 DEG C / minute, the annealing temperature is 400-700 DEG C, and the annealing time is 2-8 hours. The method can be used for preparing the high-specific-area carbon nitride material under the condition of no template, and thus, has the advantages of simplified technique, no template, one-step preparation and the like.
Owner:SHENZHEN CAPCHEM TECH CO LTD
Synthesis of decitabine
The invention discloses a synthesis method of decitabine. The synthesis method comprises the following steps: subjecting 2-deoxy-D-ribose to methoxylation in the presence of HCl, subjecting the reaction product to acylation reactions with p-methyl benzoyl chloride, coupling the reaction product with 5-azacytosine in the presence of lithium trifluoromethanesulfonate so as to obtain 1-(3,5-bi-O-p-methylbenzoyl-2-deoxy-D-ribose)-5-azacytosine, and finally subjecting the 1-(3,5-bi-O-p-methylbenzoyl-2-deoxy-D-ribose)-5-azacytosine to sodium methoxide deprotection and methanol recrystallization so as to obtain the antitumor drug decitabine, and the total yield of the synthesis method is 30.2%.
Owner:NANJING UNIV OF TECH
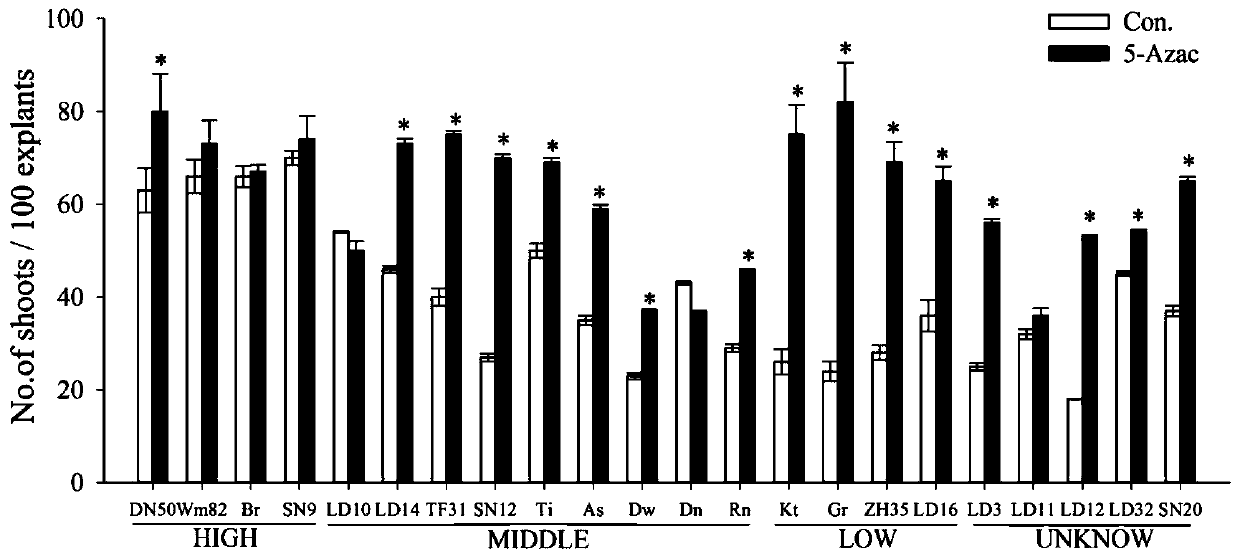
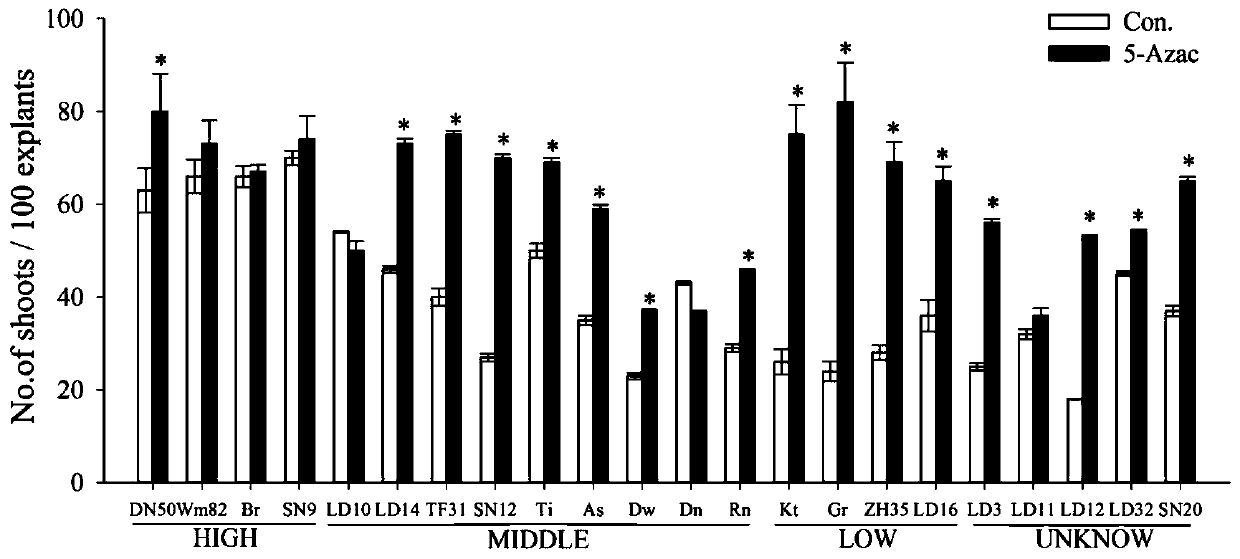
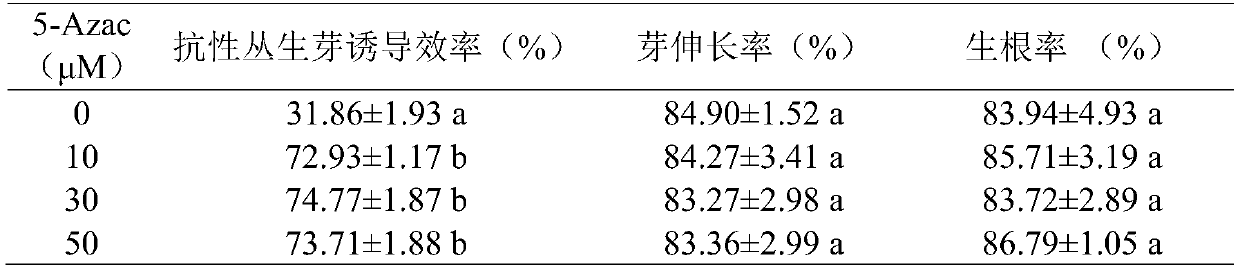
Method for improving soybean resistant cluster bud induction efficiency by using 5-azacytidine
PendingCN110042120AImprove induction efficiencyVector-based foreign material introductionTransformation efficiencyBud
The present invention relates to a method for improving the soybean resistant cluster bud induction efficiency by using 5-azacytidine, and belongs to the technical field of transgene. The main technical scheme is as follows: sterilization, germination, preparation of agrobacterium liquid, soybean cotyledon node infection by agrobacterium, and resistant cluster bud induction. In an agrobacterium-mediated soybean cotyledon node genetic transformation system, the method uses a methylation inhibitor of the 5-azacytidine for the first time for carrying out demethylation treatment on the soybean cotyledon explants which induce resistant cluster buds, improves the induction efficiency of the resistant cluster buds of the soybean cotyledon explants, and may improve the transformation efficiency ofthe T0 generation.
Owner:SHENYANG AGRI UNIV
Preparation method of azacitidine
ActiveCN110128485AMild reaction conditionsShort reaction timeSugar derivativesSugar derivatives preparationTrimethylsilyl chlorideDistillation
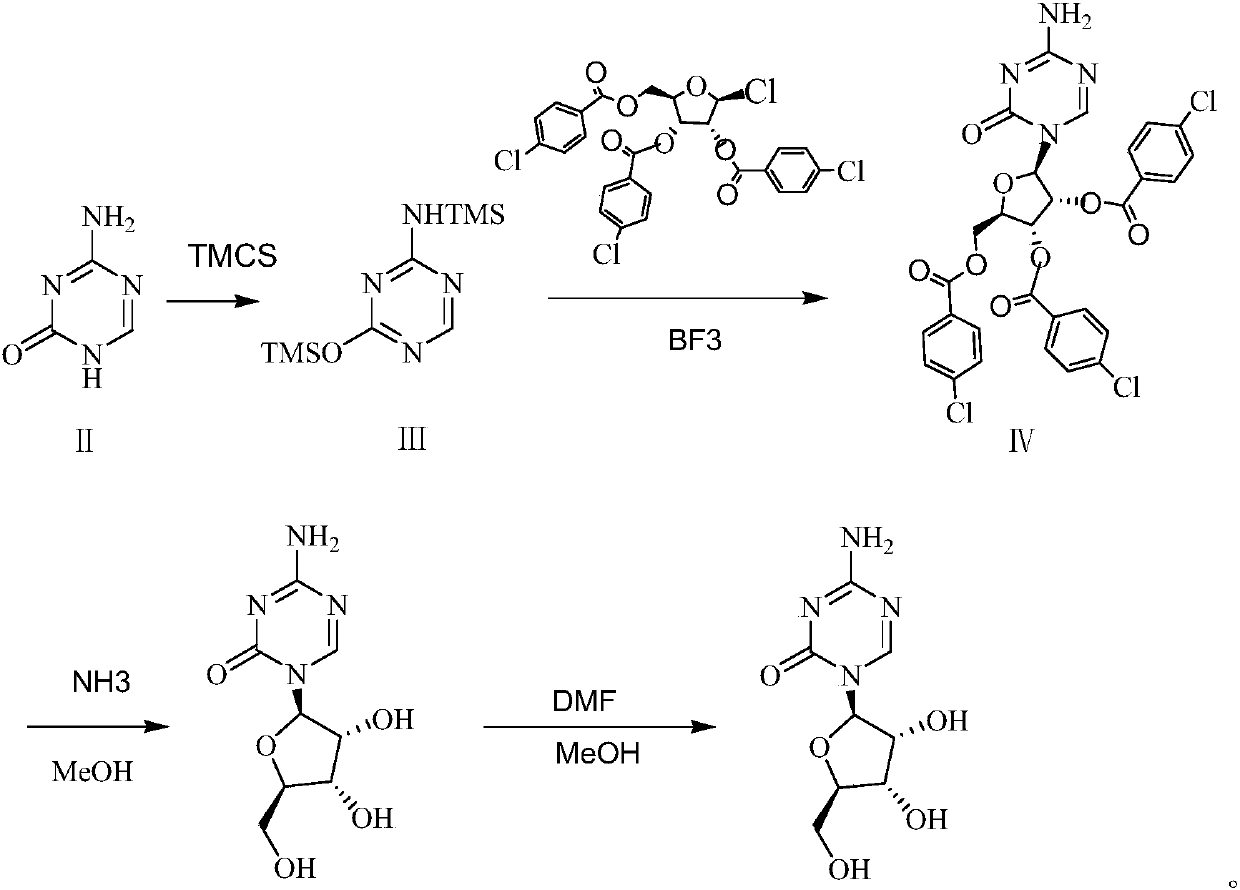
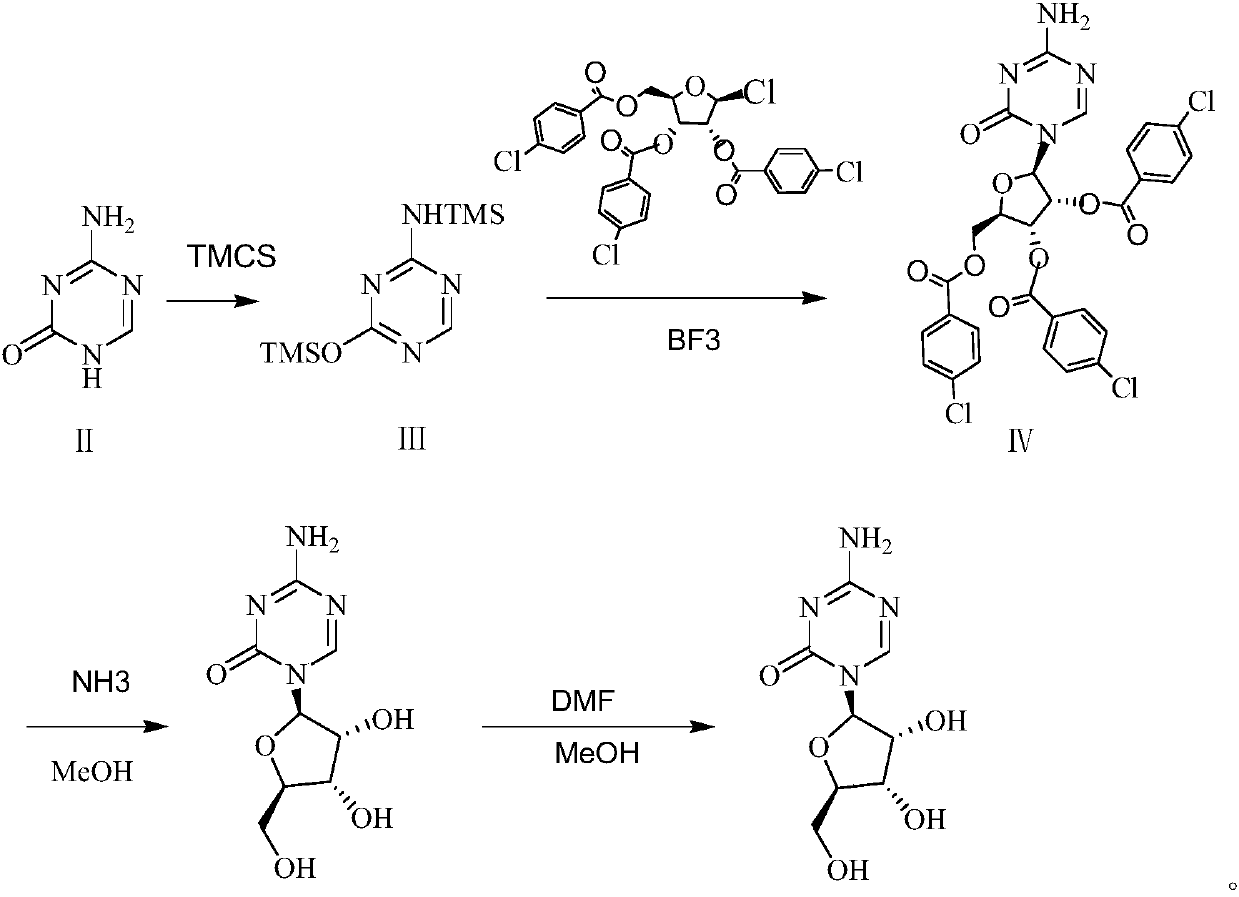
The invention belongs to the field of pharmaceutical synthesis, and specifically relates to a preparation method of azacitidine, wherein the preparation method comprises the following steps: carryingout a reaction of 5-azacytosine with trimethylchlorosilane at the temperature of 70-80 DEG C for 2 h to obtain an azacitidine intermediate I; dissolving the azacitidine intermediate I with dichloromethane, under catalysis of boron trifluoride, carrying out condensation reaction with 1-chloro-2,3,5-tri-O-p-chlorobenzoyl-beta-D-ribose, after completion of the reaction, washing, drying, carrying outsuction filtration and carrying out reduced pressure distillation of the filtrate, to obtain an azacitidine intermediate II represented by the formula IV; and carrying out alcoholysis of the azacitidine intermediate II with ammonia gas, to obtain crude azacitidine, and purifying to obtain high-purity azacitidine. The preparation method has the advantages of mild reaction conditions, short reactiontime and high yield, and is suitable for industrial production.
Owner:LUNAN BETTER PHARMA
Compositions comprising a GPR109 ligand for treating disorders of the digestive tract and/or cancer
Pharmaceutical compositions containing an effective amount of a ligand for GPR109 to decrease intracellular cAMP levels of a subject in combination with an effective amount of a DNA methyl transferase inhibito to reduce or inhibit downregulation of GPR109 in the intestinal epithelial cells of the subject relative to a control are provided. It has been discovered that ligands for GPR109 can be used to treat one or more symptoms of cancer, inflammatory disorders, and diarrhea. Representative CPR109 ligands include, but are not limited to butyrate, β-hydroxybutyrate, nicotinic acid, acifran, and octanoate. Suitable DNA methyl transferase inhibitors include 5-azacytidine, 5-aza-2′-deoxytidine, 1-β-D-arabinfarnosyl-5-azacytosine and dihydro-5-azacytidine. Typically, the compositions are formulated to achieve a GPR109 ligand serum blood level of about 1 to about 1000 μM. The compositions are useful for the treatment of one or more symptoms of cancer. Preferred cancers that can be treated using the disclosed compositions include, but are not limited to colon cancer, breast cancer and leukemia. Methods for treating cancer, inflammatory disorders, and diarrhea are also provided.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Preparation, separation and purification method of Decitabine
ActiveCN101899079BHigh purityReduce manufacturing costSugar derivativesSugar derivatives preparationPurification methodsChemical products
The invention relates to a preparation, separation and purification method of Decitabine, which adopts the following technical scheme: taking 2'-Deoxy-D-ribose and 5-Azacytosine as basic raw materials, and preparing the Decitabine by reactions of methylation, acylation, trimethyl silylation, condensation, deprotection and the like and chiral separation and purification. The method utilizes cheap chemical products as raw materials, has mild reaction conditions, adopts a simple and easy chiral separation method, and obtains a high purity product with the chemical purity of above 99.8% and optical purity of above 99.68%, thus greatly lowering production cost and being suitable for industrialized production.
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of synthetic method of 5-azacytosine
ActiveCN105837524BLower the dehydration reaction temperatureReduce generationOrganic chemistryEmulsionFormate
Owner:NINGXIA SIKEDA BIOTECH CO LTD
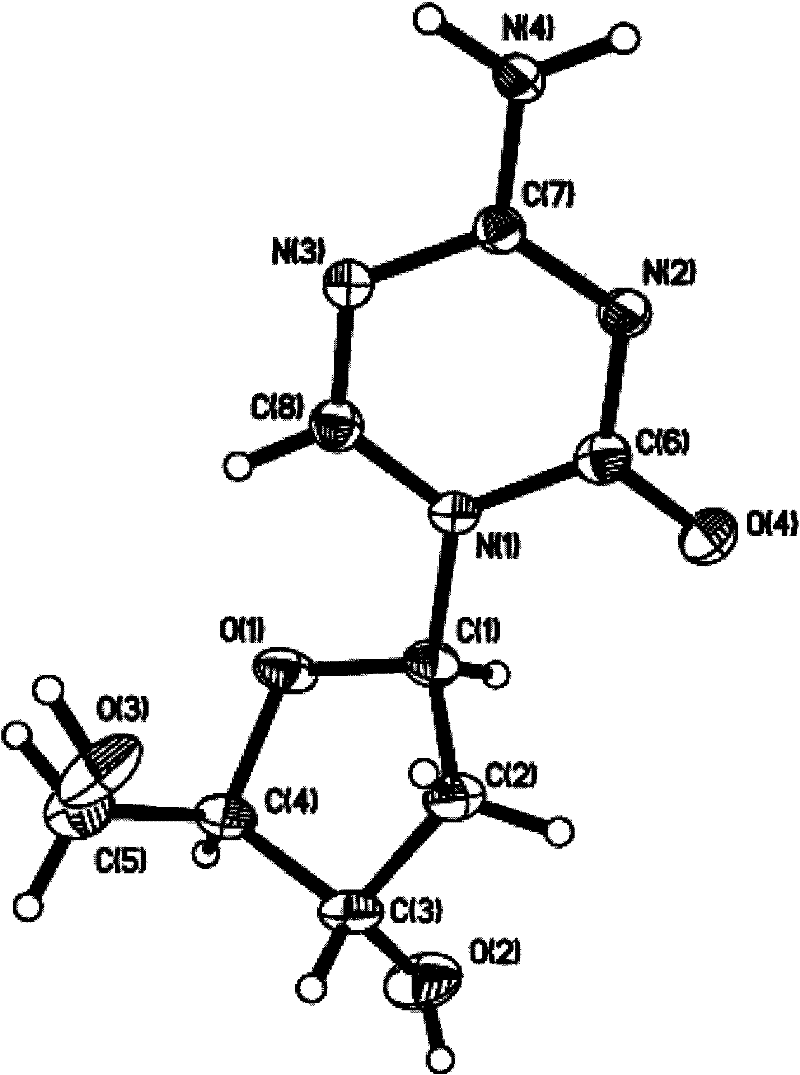
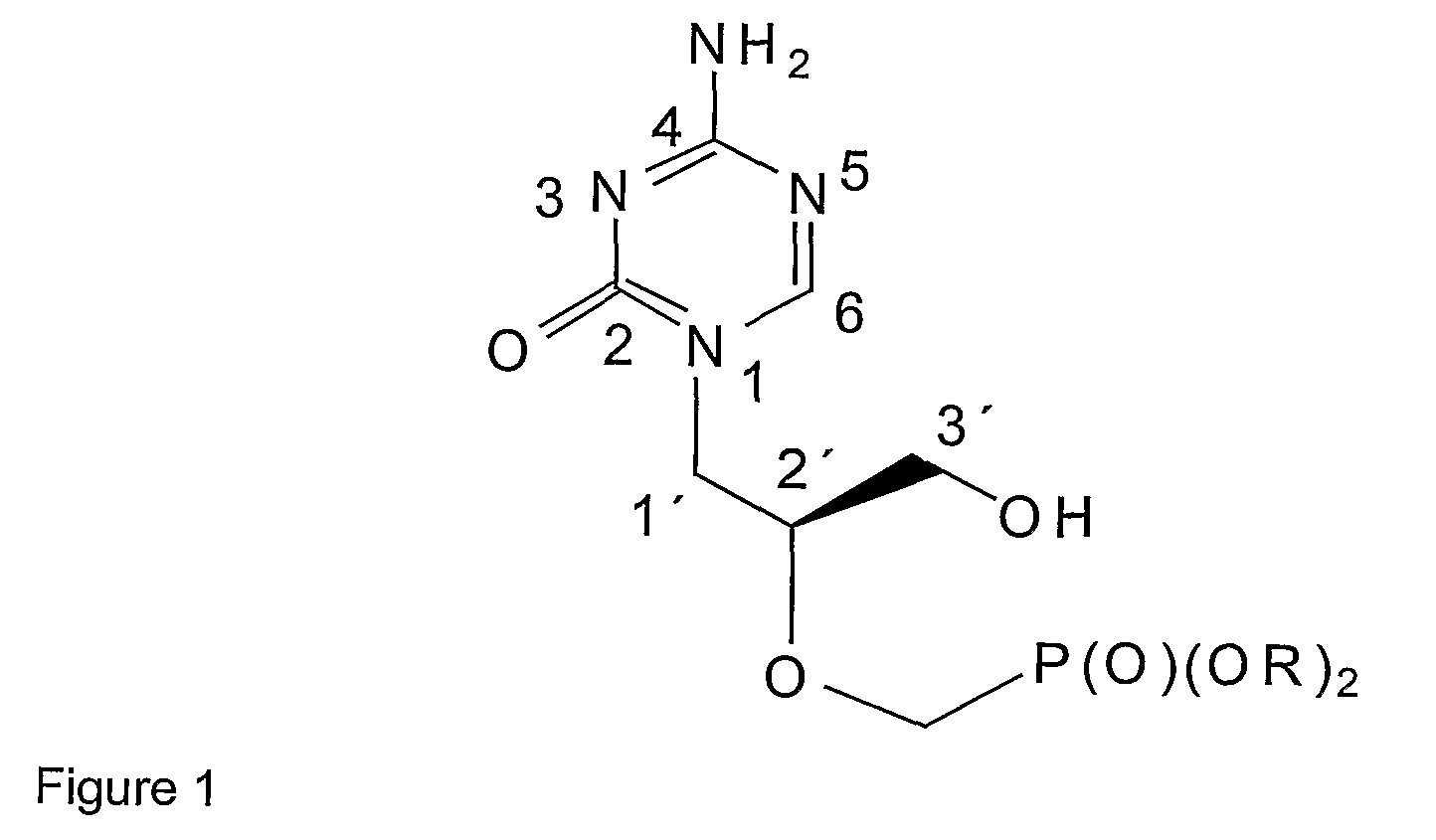
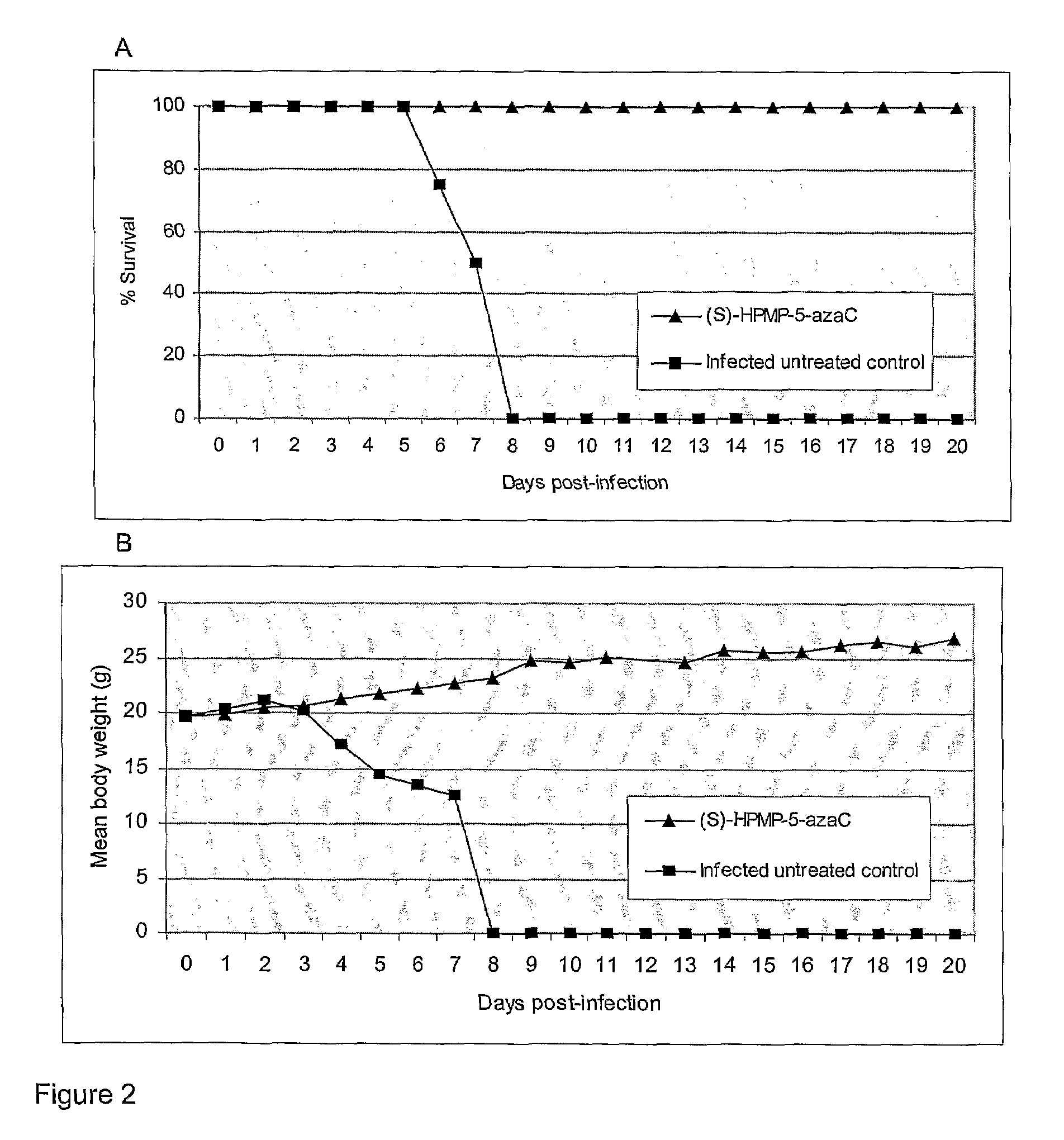
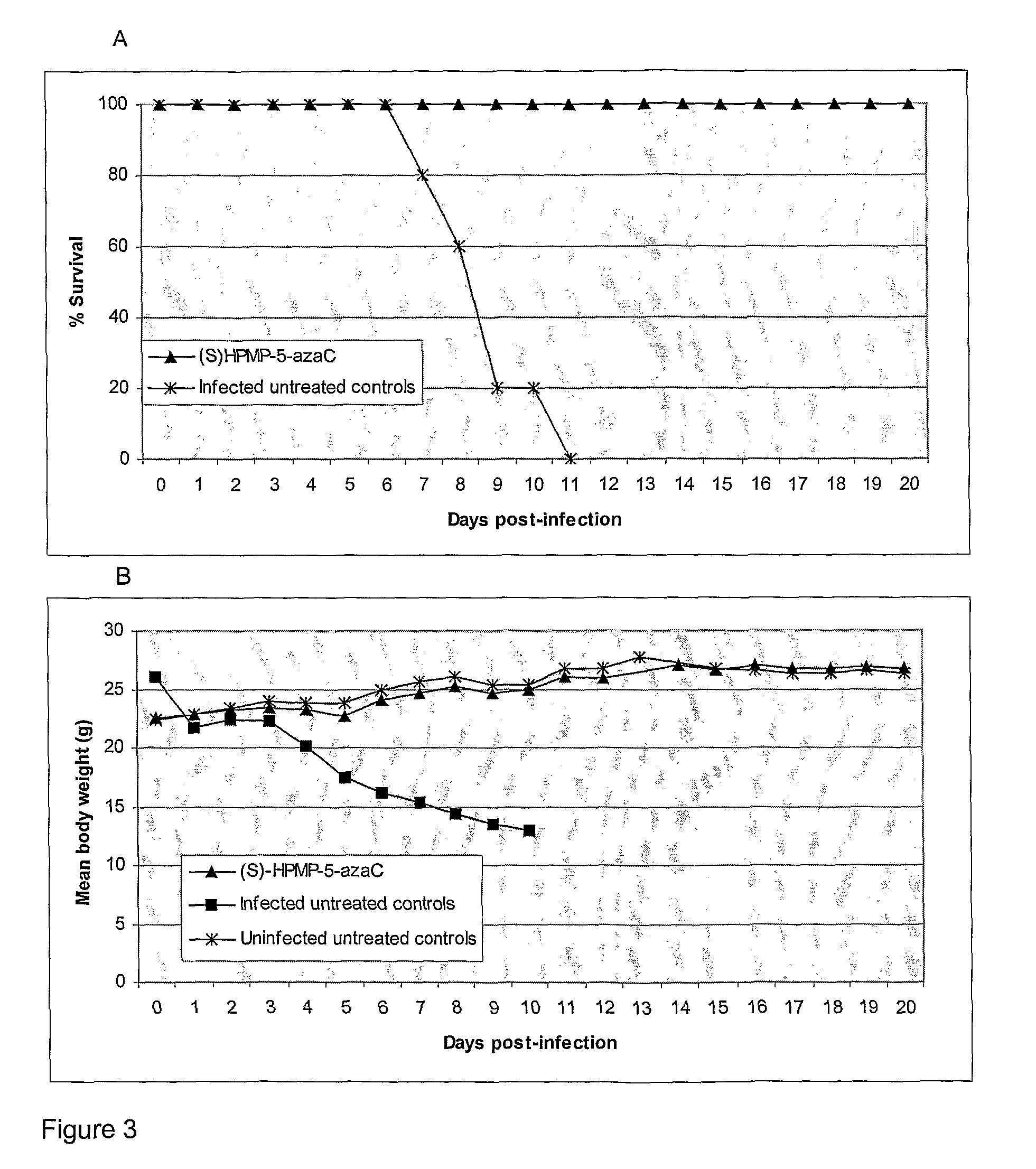
Azacytosine derivatives useful as antiviral agents
InactiveUS8153787B2Activity against virusHigh activityBiocideSugar derivativesDna viralBULK ACTIVE INGREDIENT
The present invention provides 5-azacytosine derivatives with antiviral activity, specifically having viral replication inhibiting properties, more particularly in DNA viruses such as pox-, papilloma- and herpes viruses in humans. The invention also provides pharmaceutical compositions comprising such 5-azacytosine derivatives as active ingredients in combination with pharmaceutically acceptable carriers, which are useful for the treatment of subjects suffering from viral infections.
Owner:K U LEUVEN RES & DEV +1
Preparation method of β-configuration decitabine precursor
InactiveCN107011399BStable molar yieldRaw materials are simpleSugar derivativesSugar derivatives preparationEtherLewis acid catalysis
The invention provides a preparation method of a beta-configuration decitabine precursor. The preparation method comprises the following step: under the action of a Lewis acid catalyst, directly performing a coupling reaction on acylated sugar adopting the structure of 1-alkenylmethoxy-2-deoxy-3, 5-di-O-acetyl-D-ribofuranose and a silicon ether compound of 5-azacytosine to obtain beta-configuration 1-(2-deoxy-3,5-di-O-acetyl-D-ribose)-4-amino-1,3,5-s-triazine-2-one. The beta-configuration decitabine precursor is high in selectivity, good in repeatability and very high in molar yield and purity.
Owner:ZHENGZHOU TECHN COLLEGE +1
Preparation method of beta-configuration decitabine precursor
InactiveCN107011399AStable molar yieldRaw materials are simpleSugar derivativesSugar derivatives preparationEtherLewis acid catalysis
The invention provides a preparation method of a beta-configuration decitabine precursor. The preparation method comprises the following step: under the action of a Lewis acid catalyst, directly performing a coupling reaction on acylated sugar adopting the structure of 1-alkenylmethoxy-2-deoxy-3, 5-di-O-acetyl-D-ribofuranose and a silicon ether compound of 5-azacytosine to obtain beta-configuration 1-(2-deoxy-3,5-di-O-acetyl-D-ribose)-4-amino-1,3,5-s-triazine-2-one. The beta-configuration decitabine precursor is high in selectivity, good in repeatability and very high in molar yield and purity.
Owner:ZHENGZHOU TECHN COLLEGE +1
A kind of carbon nitride and preparation method thereof
The invention discloses a preparation method of carbon nitride, which comprises the following step: carrying out high-temperature annealing on 5-azacytosine in a non-oxidizing atmosphere to prepare the carbon nitride, wherein the heating rate is 0.2-10 DEG C / minute, the annealing temperature is 400-700 DEG C, and the annealing time is 2-8 hours. The method can be used for preparing the high-specific-area carbon nitride material under the condition of no template, and thus, has the advantages of simplified technique, no template, one-step preparation and the like.
Owner:SHENZHEN CAPCHEM TECH CO LTD
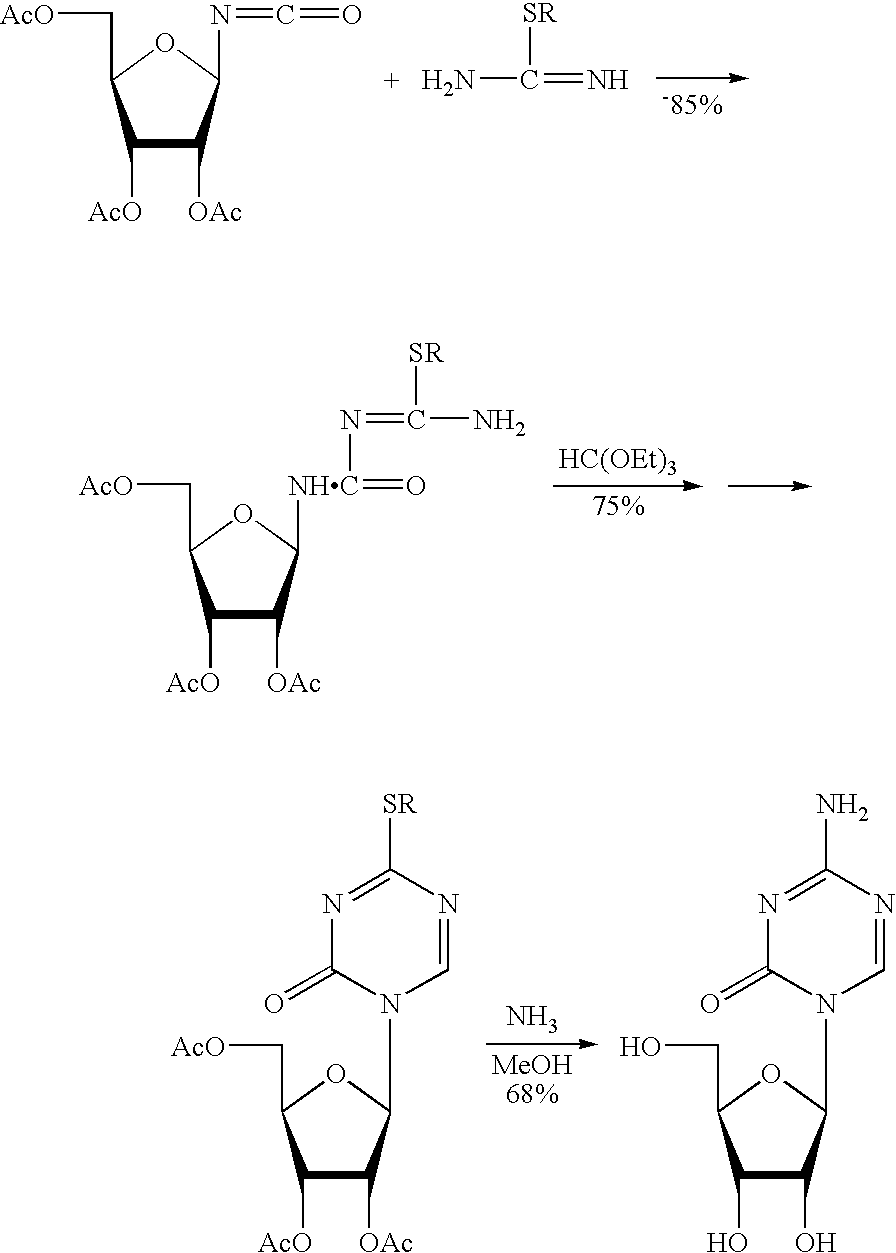
The preparation method of 5-azacytosine
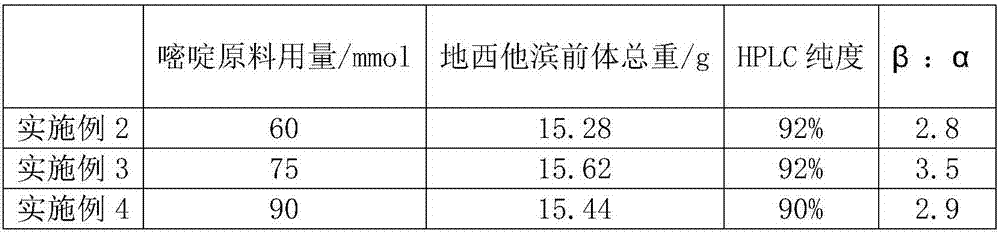
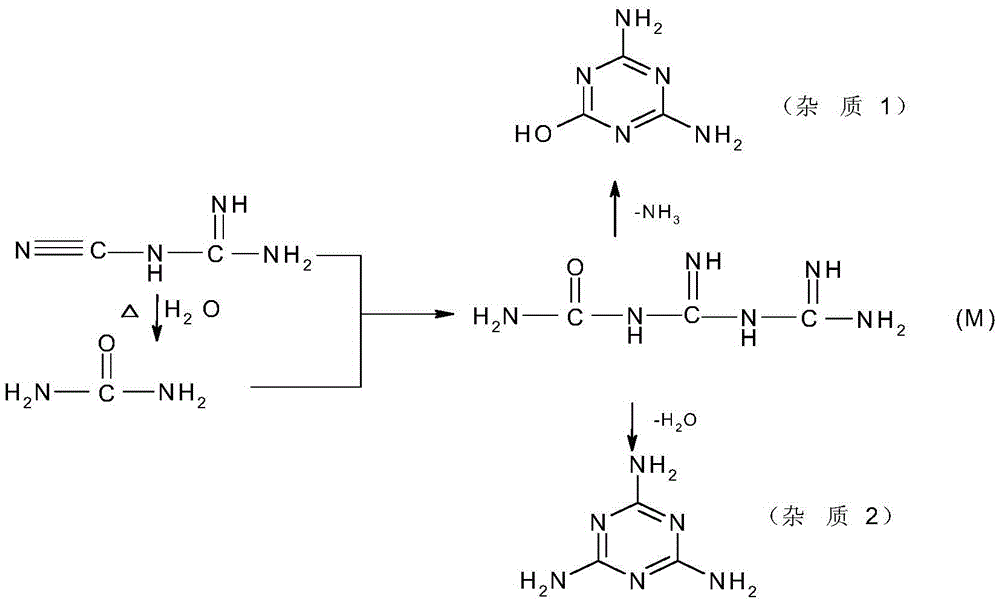
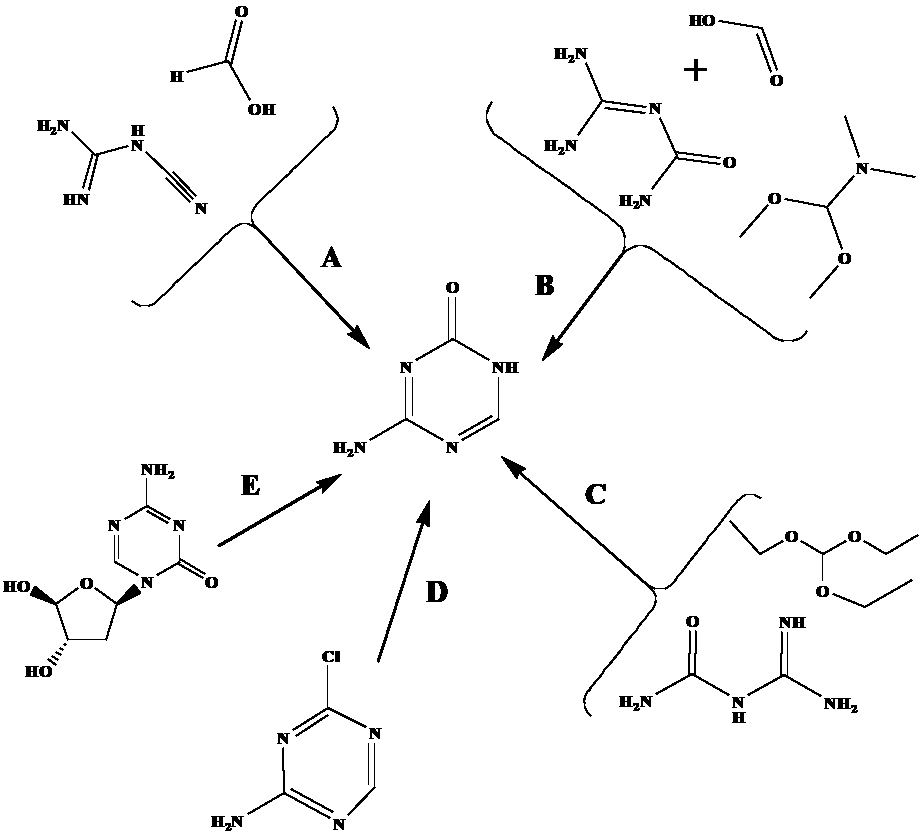
The invention relates to a preparation method for 5-azacytosine. The preparation method comprises: firstly reacting formic acid with a part of dicyandiamide at 50-60 DEG C, adding the residual dicyandiamide in batches after the reaction is steady, reacting at a constant temperature of 50-60 DEG C for 4 h, then heating to 100-110 DEG C and reacting for 1 h, adding acetic anhydride by dividing into two times, raising the temperature and performing refluxing reaction for 2 h, cooling to 60-70 DEG C, adding ethanol, continuing to cool to room temperature, filtering to obtain a 5-azacytosine crude product, then adding a mixed solution of ethyl acetate and ethanol, filtering and baking to dry, so as to obtain the 5-azacytosine finished product. The beneficial effects comprise that the prepared 5-azacytosine has the HPLC purity of 98.5% or more, has the impurity 1 content less than 0.5% and the impurity 2 content less than 0.1%, and the preparation method is good in technological safety, free of technological wastewater and suitable for large-scale industrialized production.
Owner:LIVZON GROUP CHANGZHOU KONY PHARMA
Preparation method of decitabine
ActiveCN101560233BShort reaction stepsLow costSugar derivativesSugar derivatives preparationAcetic anhydrideMethyl group
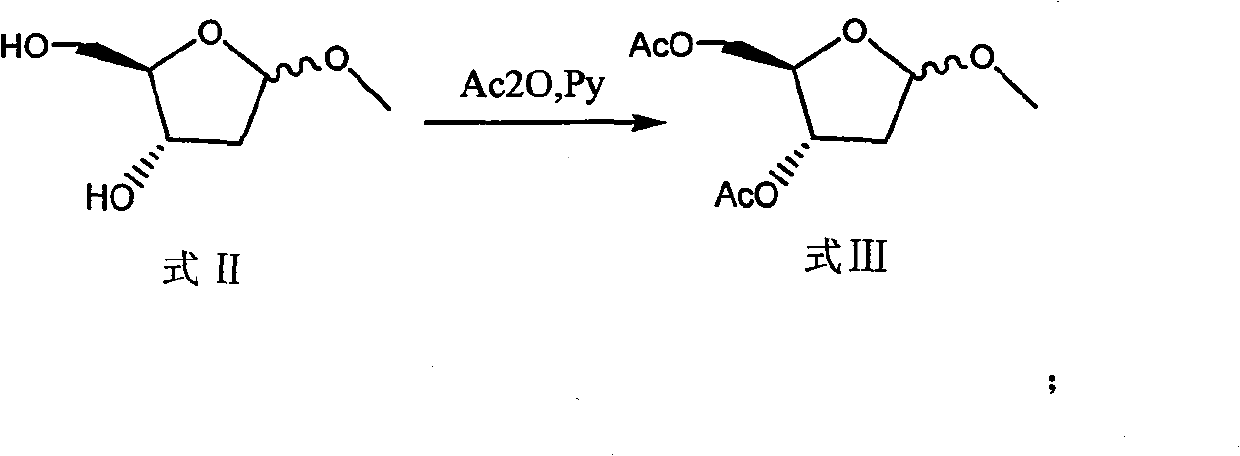
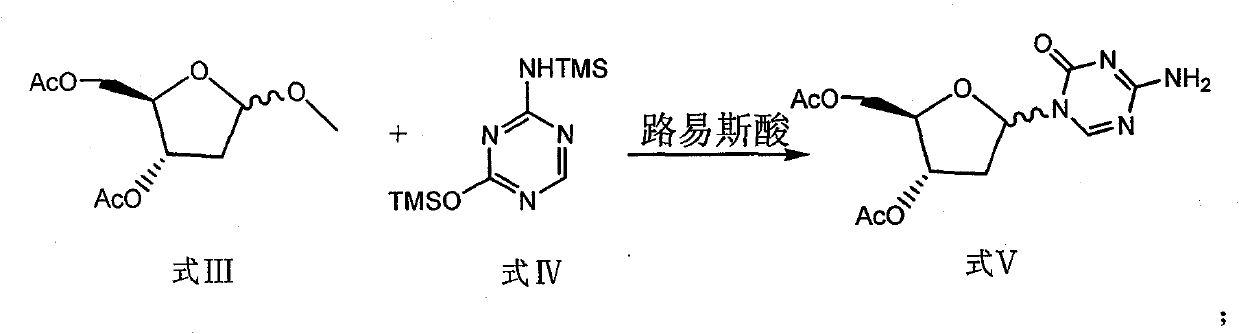
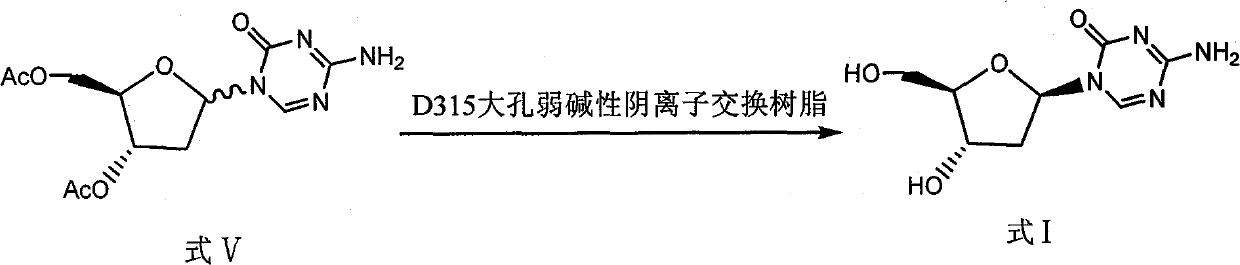
The invention discloses a preparation method of 2'-deoxycytidine analogue decitabine (formula I) capable of effectively inhibiting growth of tumour cells. The method comprises the steps of using 1-methyl-2-deoxy-D-ribose (formula II) as initial raw material, subjecting the initial raw material and acetic anhydride to a reaction to obtain 1-methyl-3,5-diacetoxyl-2-deoxy-D-ribose (formula III); subjecting the compound of formula III and 2,4-bi-(trimethyl silicon)-5-azacytosine (formula IV) to a reaction to obtain 3',5'-diacetoxylgroup-5-aza-2'-deoxycytidine (formula V); hydrolysing the compoundof formula V under the action of D315 macro-porous weakly basic anion exchange resin to obtain the decitabine (formula I). The raw materials of the invention are easily obtained, the reaction conditions are moderate and the operation is simple and convenient. The preparation method is suitable for commercial production.
Owner:SHANGHAI QINGSONG PHARMA
New synthesis method of 5-aza-cytosine
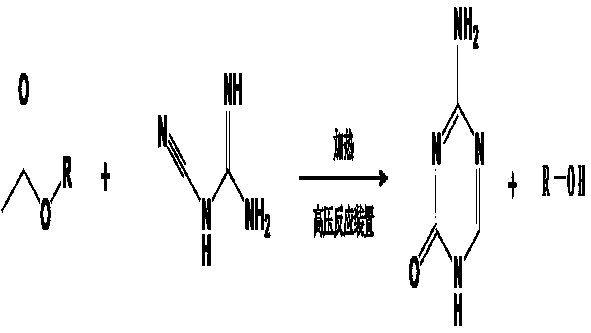
ActiveCN109020907AEasy to separate and recycleAvoid high temperature polymerization side reactionsOrganic chemistryFormateSynthesis methods
The invention discloses a new synthesis method of 5-aza-cytosine, which includes the steps of: (1) adding dicyandiamide and formate into a sealed reaction device, performing a heating reaction and distilling out non-reacted formate and corresponding alcohol to obtain a white solid; (2) purifying the white solid with hydrochloric acid and ammonia water to obtain the 5-aza-cytosine. In the method, the 5-aza-cytosine is directly synthesized through a reaction on the dicyandiamide and formate in a high-pressure reaction kettle, wherein the formate serves as a reactant, a reaction solvent, and a dehydrator in a ring-closing reaction in the process, so that processes of hydrolysis of the formate, hydration of the dicyandiamide and dehydration of dicyandiamidine formate can be carried out simultaneously under a hydrothermal condition, thus simplifying the synthesis route, avoiding side reactions and increasing purity of product. The total reaction yield is higher than 70% on the basis of dicyandiamide, and product purity is higher than 98%.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com