Azacitidine for injection and preparation method thereof
A technology for azacitidine and injection, which is applied in the field of medicine and can solve the problems of clinical drug safety and effectiveness, poor product stability, and difficult control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1.1 Prescription
[0040]
[0041]
[0042] 1.2 Freeze-drying procedure
[0043]
[0044] 1.3 Preparation method
[0045] (1) Measure 80% water for injection, add mannitol, and stir until completely dissolved;
[0046] (2) Add activated carbon, keep warm in 80°C water bath for 20 minutes, and filter to remove carbon;
[0047] (3) Make up the whole amount with water for injection, cool down to -1~-3°C, and adjust the pH to 6;
[0048] (4) Add azacitidine, stir until completely dissolved, and strictly control the temperature at -1~-3°C;
[0049] ⑸Filling, 25ml / bottle;
[0050] ⑹ Turn on the freeze dryer, and after pre-freezing at -50°C for 3 hours, turn on the vacuum pump and run the program;
[0051] ⑺Vacuum plugging, capping and packaging.
[0052] 1.4 Full inspection results
[0053] 1.4.1 Moisture: 1.25%±0.27 (n=6)
[0054] 1.4.2 Content: 100.12%±0.35(n=3)
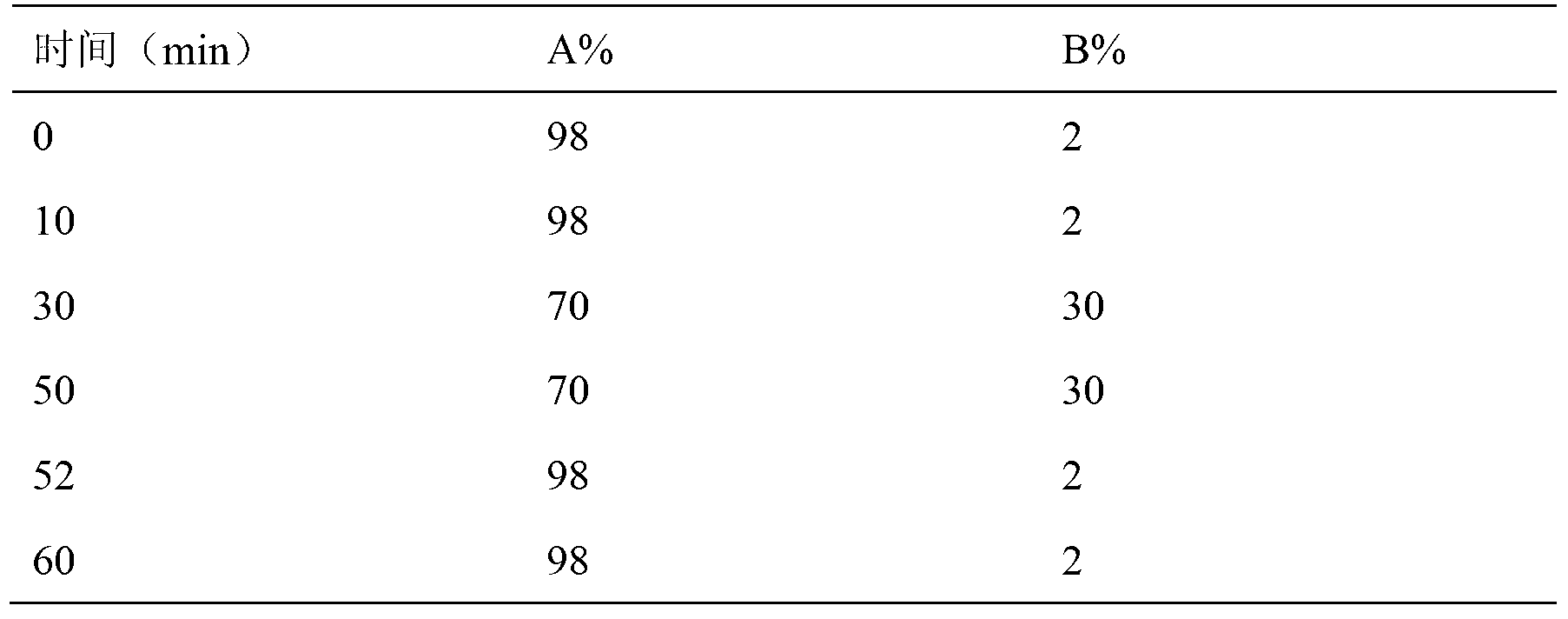
[0055] 1.4.3 Related substances:
[0056]
Embodiment 2
[0058] 2.1 Prescription
[0059]
[0060] 2.2 Freeze-drying procedure
[0061]
[0062] 2.3 Preparation method
[0063] (1) Measure 80% water for injection, add mannitol, and stir until completely dissolved;
[0064] (2) Add activated carbon, keep warm in 80°C water bath for 20 minutes, and filter to remove carbon;
[0065] (3) Make up the full amount with water for injection, cool down to -1~-3°C, and adjust the pH to 6.5;
[0066] (4) Add azacitidine, stir until completely dissolved, and strictly control the temperature at -1~-3°C;
[0067] ⑸Filling, 25ml / bottle;
[0068] ⑹ Turn on the freeze dryer, and after pre-freezing at -50°C for 3 hours, turn on the vacuum pump and run the program;
[0069] ⑺Vacuum plugging, capping and packaging.
[0070] 2.4 Full inspection results
[0071] 2.4.1 Moisture: 0.24%±0.13(n=6)
[0072] 2.4.2 Content: 99.24%±0.27(n=3)
[0073] 2.4.3 Related substances:
[0074]
Embodiment 3
[0076] 3.1 Prescription
[0077]
[0078] 3.2 Freeze-drying procedure
[0079]
[0080]
[0081] 3.3 Preparation method
[0082] 1. Measure 80% water for injection, add mannitol, stir until completely dissolved;
[0083] 2. Add 0.1% activated carbon, stir in an 80-degree water bath for 20 minutes, and filter to remove carbon;
[0084] 3. Make up the full amount with water for injection, cool down to -1~-3°C, and adjust the pH to 7;
[0085] 4. Add azacitidine, stir until completely dissolved, and strictly control the temperature at -1~-3°C;
[0086] 5. Dispensing, 25ml / bottle;
[0087] 6. Turn on the freeze dryer, and after pre-freezing at -50°C for 3 hours, turn on the vacuum pump and run the program;
[0088] 7. Vacuum plugging, capping and packaging.
[0089] 3.4 Full inspection results
[0090] 3.4.1 Moisture: 2.29%±0.26(n=6)
[0091] 3.4.2 Content: 99.75%±0.56(n=3)
[0092] 3.4.3 Related substances:
[0093]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com