Preparation method of 2-substituted arylethenyl-N-methylated quinoline derivative and application of 2-substituted arylethenyl-N-methylated quinoline derivative in preparation of drug for treating Alzheimer disease
A technology for methylating quinoline and arylvinyl, which is applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve the problem that the pathogenesis is not fully understood, and achieve the effect of inhibiting Ab aggregation, high medical value, and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
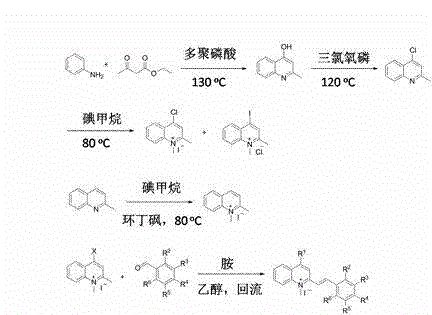
[0031] Example 1: Synthesis of 2-methyl-4-hydroxyquinoline (compound 1)
[0032] Mix 14.5 g (155.3 mmol) aniline with 20.2 g (55.3 mmol) ethyl acetoacetate, add PPA and heat to 90 oC After 2 hours, the reaction was carried out at 130°C for 2 hours. The reaction mixture was then poured into water while hot to hydrolyze the excess PPA. The pH value was adjusted to neutral with hydrochloric acid and a solid was precipitated, which was collected by filtration to obtain a yellow solid. Yield: 77%;
[0033] 1 H NMR (400 MHz, DMSO- d 6 ) δ: 11.58 (s, 1H), 8.02 (d, 1H, J = 8.0 Hz), 7.61-7.56 (m, 1H), 7.560-7.47 (m, 1H), 7.27-7.23 (m, 1H), 2.33(s, 3H). 13 C NMR (100 MHz, DMSO- d 6 ) δ: 176.67, 149.58, 140.08, 131.35, 124.73, 124.46, 122.61, 117.69, 108.33, 19.40; MS (ESI + APCI) m / z: 160.1 [M+H] +1 .
[0034]
Embodiment 2
[0035] Example 2: Synthesis of 2-methyl-4 chloroquinoline (compound 2)
[0036] Mix 2.5 g (88.9 mmol) 2-methyl-4-hydroxyquinoline and 125 mL phosphorus oxychloride (POCl3) at 120 o C reacted for 2h. Then, the reaction mixture was poured into water to hydrolyze excess POCl3 while still hot, and the pH was adjusted to neutrality with hydrochloric acid to obtain a gray solid, which was collected by filtration.
[0037] 1 H NMR (400 MHz, DMSO-d6) δ: 8.14 (d, 1H, J = 8.0 Hz), 8.02 (d, 1H, J= 8.0 Hz), 7.72-7.68 (m, 1H), 7.56-7.52 (m, 1H), 7.34 (s, 1H), 2.69 (s, 3H). 13 C NMR (100 MHz, DMSO- d 6 ) δ: 153.53, 143.27, 137.30, 125.10, 123.58, 121.38, 119.41, 118.60, 116.63, 19.78; MS (ESI + APCI) m / z: 179.1 [M+H] +1 .
[0038]
Embodiment 3
[0039] Example 3: Synthesis of 1,2-dimethyl-4-chloro (iodo) quinoline iodide (chloro) compound (compounds 3, 4)
[0040] 28.0 g of intermediate 2-methyl-4-chloroquinoline derivative (compound 2), 50 mL of sulfolane and 11.2 mL of methyl iodide were mixed and sealed in a 250 mL sealed tube, and then placed in 80 o The reaction was carried out in the oil bath of C overnight, and a large amount of purple solid appeared in the reaction solution. Stop the reaction and cool down to remove excess methyl iodide (Note: methyl iodide is highly toxic, be careful not to inhale or touch the skin when removing), then add 250 mL of anhydrous ether was stirred, the sulfolane was removed by suction filtration, and finally washed with anhydrous ethanol to obtain a yellow solid. The yield is 90%;
[0041] 3: 1 H NMR (400 MHz, DMSO- d 6 ) δ: 8.67 (d, 1H, J = 8.0 Hz), 8.56 (d, 1H, J = 12.0 Hz ), 8.54 (s, 1H,), 8.33 (t, 1H, J = 8.0 Hz), 8.12 (t, 1H, J = 8.0 Hz), 4.44 (s, 3H), 3.08 (s, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com