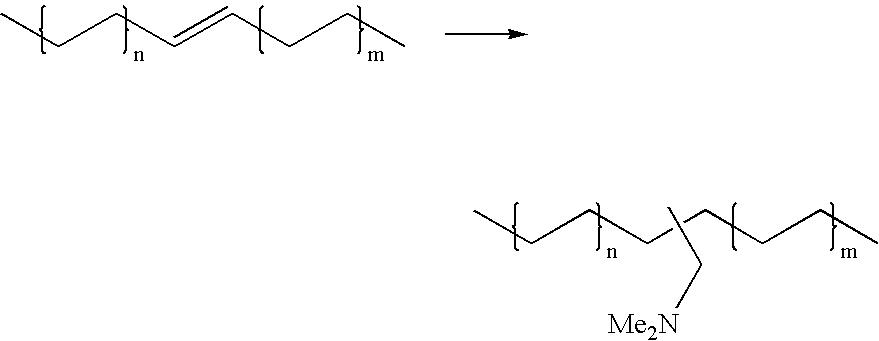
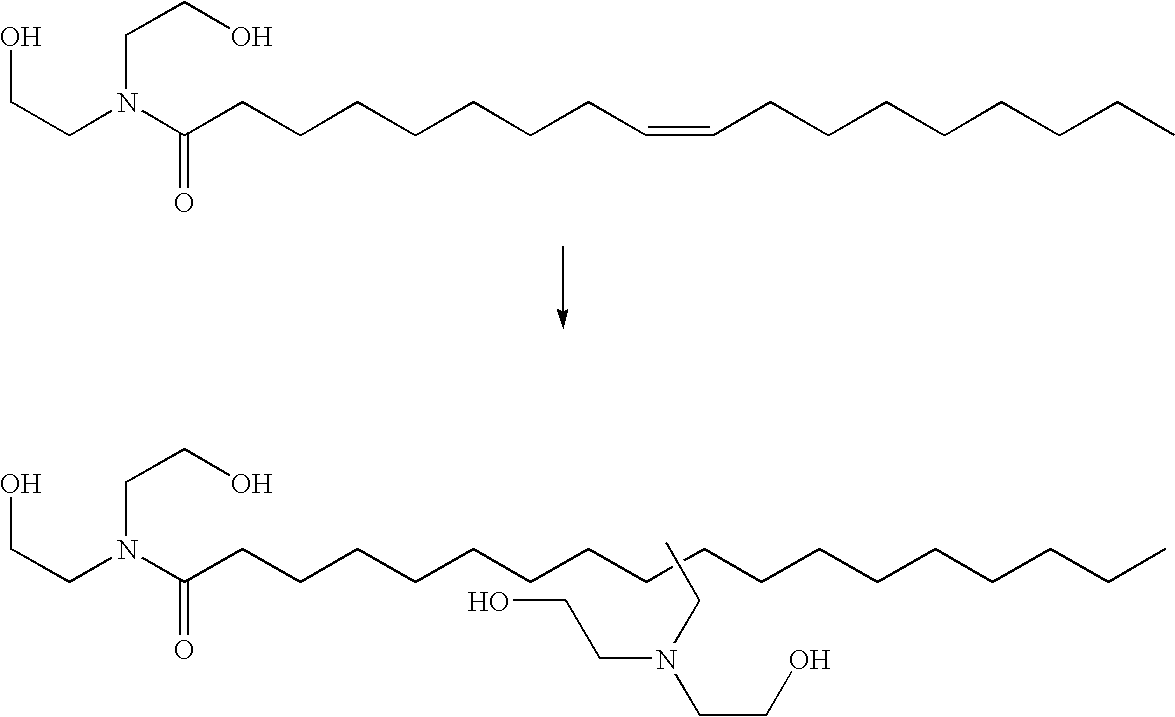
Hydroaminomethylation of olefins
a technology of olefins and methylated olefins, which is applied in the field of phosphite ligands less suitable for the desired reaction, poor amine selectivity, and no conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Dimethylaminomethylalkane mixture
[0022]
[0023] Neodene® Olefin Blend (10 / 1112 / 13 / 12, 319 g, obtained from Shell Chemical) olefin substrate was added to a 1-gal (4-L) reactor in THF (680 g). To this solution was added dimethylamine (222 g), and a solution of a complex prepared from [Rh(CO)2(acac)] (3.7 g) and tris(2,4-di-t-butylphenyl)phosphite (46.2 g, obtained from Aldrich Chemical) in THF (374 g). The final rhodium concentration was about 900 ppm by weight. This mixture was heated to 80° C. and pressurized to 600 psi (4140 kPa) with syngas and stirred under these conditions for 7 hours. The reactor was cooled to 21° C. overnight and the gas phase vented. After stirring under a nitrogen flow for about 1 hour to remove most of the excess dimethylamine, the reactor contents were dumped, stripped of THF and distilled using a Kugelrohr apparatus to yield a colorless mobile liquid (427 g, 99% yield).
example 2
Hydroaminomethylation of Oleic Acid Diethanolamide with Diethanolamine
[0024]
[0025] Oleic acid amide (13.49 g), diethanolamine (4.51 g) and THF solvent (20 mL) were added to a nitrogen purged reactor by syringe. The reactor was stirred under ca. 200 psi (1380 kPa) of syngas to saturate the solution. After venting, an aliquot (10.4 g) of a pre-made catalyst solution of [Rh(CO)2(acac)] (5351 ppm by weight Rh) and tris(2,4-di-t-butylphenyl)phosphite ligand (5.00 moles of ligand / mole of Rh) in THF was added to the reactor. The reactor was sealed and pressurized with syngas and heated to 80° C. The pressure was increased to 600 psi (4140 kPa) with additional syngas, and then fed on demand at this pressure throughout the reaction. After 4.5 hours, the reactor was cooled, vented and the reactor contents discharged. The amber solution was extracted with cyclohexane, then toluene, and the supernatent discarded. Acetonitrile was added to the lower brown layer, and stirred briefly. When agitat...
example 3
Hydroaminomethylation of Terminally Unsaturated Isopolypropylene with Dibenzylamine
[0026]
[0027] Vinylidene-terminated polypropylene having a weight average molecular weight Mw of about 1800 g / mol (5.43 g, obtained from Baker-Hughes Corporation) was dissolved in toluene (70 mL). A portion of this solution (65 mL) was transferred to a 100 mL Parr reactor, whereupon dibenzylamine (2 mL, 9.8 mmols) and a solution containing complex (20 mL of a stock solution prepared by dissolving [Rh(CO)2(acac)] (2.56 g), 2,4-di-t-butylphenylphosphite (32.09 g), in sufficient THF to give a total solution mass of 200.73 g) was added. The reactor was sealed and pressurized to 600 psi (4140 kPa) of syngas and heated to 80° C. for 130 minutes. After reaction, the pressure was released and the reactor opened while the temperature was still at 60° C., and the contents discharged into a glass beaker. The polymer product was precipitated by addition of methanol (about 2× volume). The solid material was filter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com