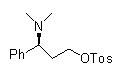
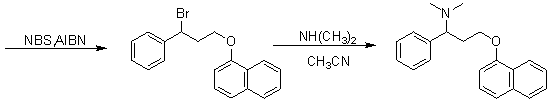
Novel synthesizing method of dapoxetine
A technology of dapoxetine and a synthesis method, applied in a new synthesis field, can solve the problems of complicated operation, unfavorable industrial production, high price and the like, and achieves the effects of cheap raw materials, low cost and simple separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0058] In order to make the technical means, creative features, work flow, and use methods of the present invention achieve the purpose and effect easily understood, the present invention will be further described below in conjunction with specific embodiments.
[0059]
[0060] I
[0061]
[0062] II III
[0063]
[0064] III IV
[0065]
[0066] V
[0067]
[0068] VI VII
[0069] Synthesis of chiral catalyst (s)-α,α-diisopropyldimethyl-tert-butylsilyloxyprolinol:
[0070]
[0071] 1
[0072]
[0073] 2 3
[0074] Synthesis of Intermediate 1:
[0075] Add 31.2 grams of magnesium powder (1.30mol) and 50 milliliters of methyl tert-butyl ether into the reaction flask, under stirring, under nitrogen protection, slowly add 147.6 grams of 2-bromopropane (1.20mol) in methyl tert-butyl ether 500ml of ether solution (15ml will be added dropwise first, and after the reaction is triggered, slowly add the remaining solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com