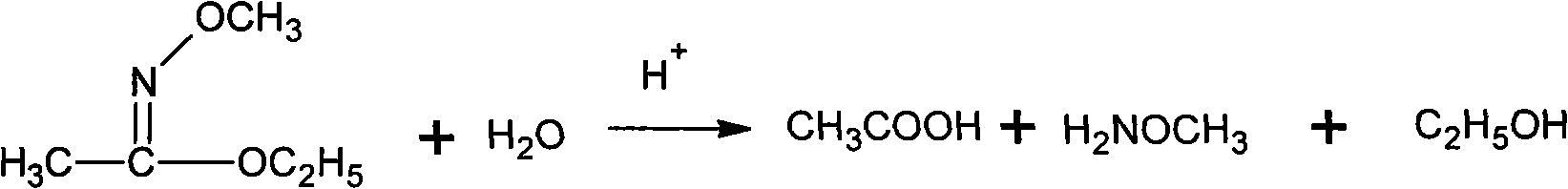
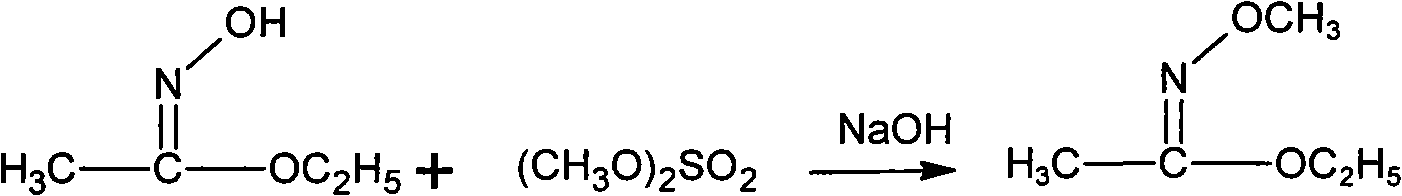Method for synthesizing methoxamine hydrochloride
A technology of methoxyamine hydrochloride and methoxyamine, which is applied in directions such as organic chemistry, can solve problems such as yield decline, side reactions, high side reactions, etc., and achieves improved product yield and quality, good atom economy, Good operating environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 10.6g (0.12mol) of ethyl acetate and 7.0g (0.10mol) of hydroxylamine hydrochloride into a 100mL flask equipped with an electric stirrer, a thermometer, and a condenser tube, cool to 10°C, and start to drop 40g of NaOH with a mass fraction of 20%. (0.2mol) aqueous solution, added dropwise for 1 h, and reacted at 15°C for 0.5 h after the dropwise addition. Then, 15.1 g (0.12 mol) of dimethyl sulfate was added dropwise at this temperature, and then the temperature was raised to 70° C. for 4 h. Cool to 10°C, add 200mL of cold water, then extract three times with 100mL of chloroform, and combine the extracts. The solvent was distilled off under reduced pressure (0.05 MPa) at 30°C, and the distillation residue was added to 17g of hydrochloric acid with a mass fraction of 15%, and the temperature was raised to 65°C, and kept for 0.5h. Then cool to 10°C, add dropwise a mass fraction of 30% sodium hydroxide solution at no more than this temperature to adjust the pH to 11, t...
Embodiment 2
[0042] The amount of ethyl acetate was changed to 0.14mol, and the others were the same as in Example 1. The result was methoxyamine hydrochloride 7.5g, a yield of 89.8%, and a product mole fraction of 99.2%.
Embodiment 3
[0044] 24g of 25% NaOH (0.15mol) solution was added dropwise for oximation, and the others were the same as in Example 1. The result was 7.2g of methoxyamine hydrochloride, the yield was 86.2%, and the product mole fraction was 98.6%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com