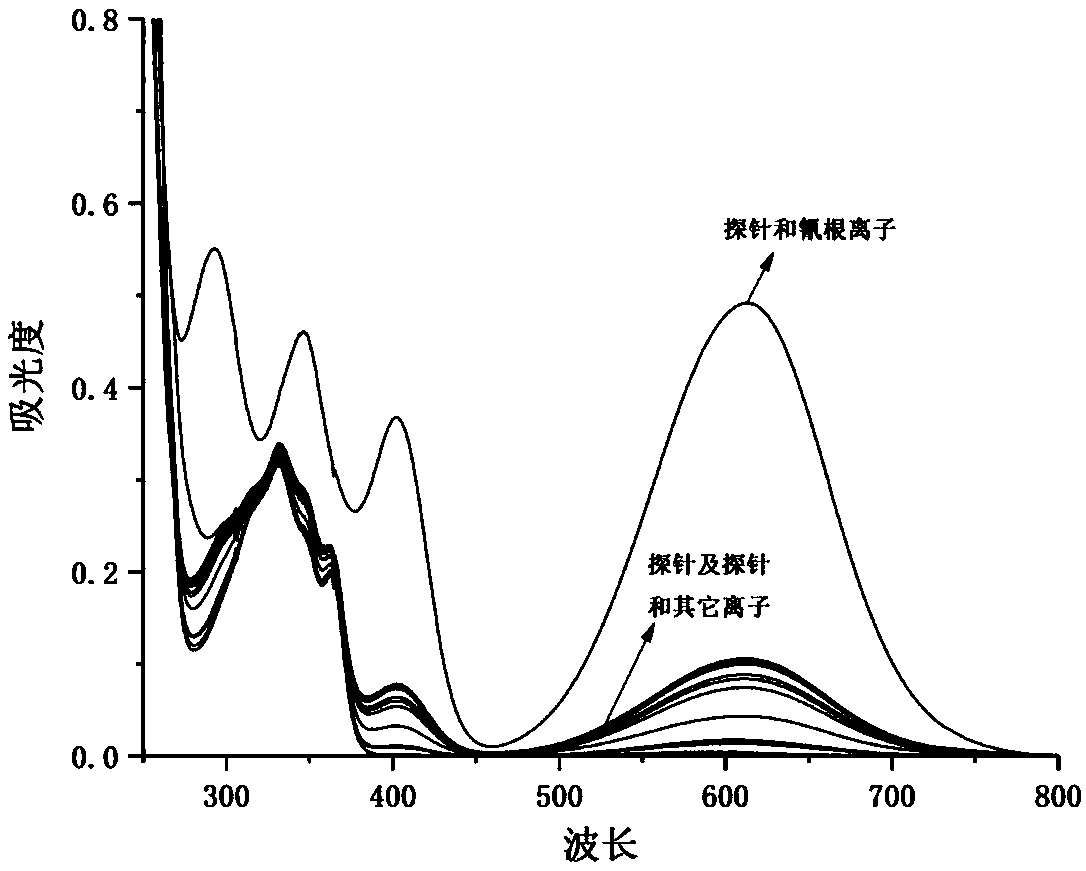
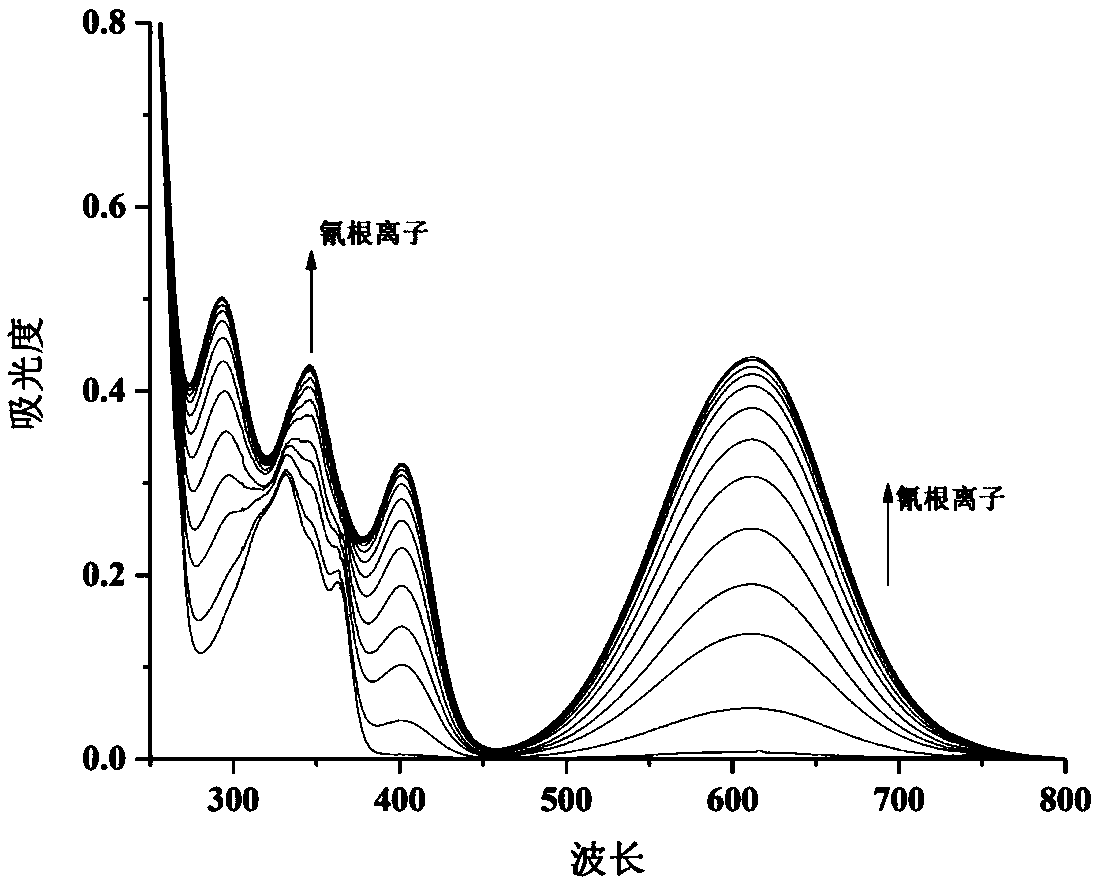
4-trifluoromethyl-6-bromo-2-substituted acetonitrile-1,8-naphthalimide compounds as well as preparation method and application thereof
A technology of naphthalimide and trifluoromethyl, which is applied in the field of organic photoelectric or fluorescent probes, can solve the problems that there are not many naphthalimide derivatives, and there are no reports on naphthalimide derivatives. Ultra-high sensitivity and selectivity, low production cost, and simple synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A 4-trifluoromethyl-1,8-naphthalimide compound, its structural formula is as follows:
[0045]
[0046] where R 1 is n-butyl;
[0047] The reaction equation of its synthetic process is as follows:
[0048]
[0049] The concrete steps of its preparation method are as follows:
[0050] In a 50ml one-mouth bottle, first add 2.0g (6.04mmol) N-n-butyl-4-bromo-1,8-naphthalimide and cuprous iodide CuI (1.72g (9.04mmol), then add 40ml The organic solvent DMF, and finally add 2.54g (13.24mmol) of methyl fluorosulfonyl difluoroacetate. Under magnetic stirring, keep the temperature at 85-90°C for 20h to obtain the above-mentioned 4-trifluoromethyl-1,8-naphthalene Amine compounds;
[0051] After the reaction is completed, first filter to remove the insoluble matter, add ethyl acetate to the filtrate, then wash the organic phase with a large amount of water and saturated saline three times, then dry it with anhydrous sodium sulfate, and then carry out chromatography on a s...
Embodiment 2
[0059] A 4-trifluoromethyl-6-bromo-1,8-naphthalimide compound, the structural formula of which is as follows:
[0060]
[0061] where R 1 is n-butyl;
[0062] The reaction formula of its synthetic process is as follows:
[0063]
[0064] The concrete steps of its preparation method are as follows:
[0065] In a 50ml single-mouth bottle, add 1.0g (3.11mmol) of 4-trifluoromethyl-1,8-naphthalimide compound obtained in the above-mentioned Example 1, that is, N-n-butyl-4-trifluoroform Base-1,8-naphthalimide, 20ml concentrated sulfuric acid. Add 0.55g (3.11mmol) of N-bromosuccinimide (NBS) under ice bath and stir for 10min, remove the ice bath and continue to stir for 1h at room temperature, and track the reaction process with TLC until the reaction is complete;
[0066] After the reaction was completed, the reaction solution was poured into ice water, extracted with ethyl acetate, and then the organic phase was washed with saturated sodium bicarbonate solution, water and ...
Embodiment 3
[0074] A 4-trifluoromethyl-6-bromo-2-substituted acetonitrile-1,8-naphthalimide compound, the structural formula of which is as follows:
[0075]
[0076] where: R 1 is n-butyl; R 2 For phenyl.
[0077] The reaction formula of its synthetic process is as follows:
[0078]
[0079] The concrete steps of its preparation method are as follows:
[0080] Add 0.17g (1.41mmol) phenylacetonitrile, 0.28g (7.0mmol) base catalyst NaH (60w%) and tetrahydrofuran 25ml in the there-necked flask of 50ml, nitrogen replacement three times, add the 0.5g ( 1.25 mmol) of N-n-butyl-4-trifluoromethyl-6-bromo-1,8-naphthalimide continued to react at room temperature for 3 hours, and the reaction process was tracked by TLC until the reaction was complete;
[0081] After the reaction, the pH of the reaction liquid was adjusted to 1-2 with a saturated citric acid solution, and then extracted with ethyl acetate. The organic phase obtained was washed 3 times with saturated brine and then dried w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com