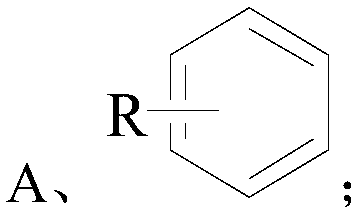Preparation method and application of aryl trifluoromethyl compound
A technology of aryl trifluoromethyl and trifluoromethyl, which is applied in the field of preparation of aryl trifluoromethyl compounds, can solve the problems of complex products in the process, catalyst residue, harsh preparation conditions, etc., and achieve the effect of simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The embodiment of the present invention provides a preparation method of an aryl trifluoromethyl compound, which at least includes the following steps:
[0033] Under light conditions and alkaline conditions, compound A and a trifluoromethyl reagent are reacted in an organic solvent system containing a catalyst to obtain an aryl trifluoromethyl compound;
[0034] Wherein the structural formula of compound A is as follows:
[0035]
[0036] The R in the structural formula of the compound A is one or more of alkyl, halogen, nitro, alkoxy, hydroxyl, and carboxyl.
[0037] The preparation method of the aryl trifluoromethyl compound of the present invention will be further explained in detail below.
[0038] In the light conditions of the present invention, the preferred light wavelength is 430nm-550nm. Under the light irradiation of this wavelength, the catalysis of the transition metal copper or palladium complex is not required, and no specific functional group is use...
Embodiment 1
[0060] This embodiment provides a preparation method of 4-trifluoromethylphenol. The structural formula of this 4-trifluoromethylphenol is as shown in molecular structural formula I1:
[0061]
[0062] Its preparation steps are as follows:
[0063] Into a dry and clean reaction vessel, add 1mmol phenol, 2mmol CF 3 SO 2 Cl, 2 mL of MeCN, 2 mmol K 2 HPO 4 , while adding 0.05mmol of acridinium salt Under white light irradiation, stir for 24h.
[0064] After the reaction was completed, the reaction was also poured into water, extracted with ethyl acetate, and extracted three times repeatedly, the organic phases were combined, dried over anhydrous sodium sulfate, and the organic phase was concentrated to obtain a crude product, which was separated by column chromatography to obtain a purified product. The product was colorless Liquid, 87% yield.
[0065] Correlation characterization analysis, the results are: 13C NMR (CDCl3, 200MHz, ppm) delta 157.8, 127.3, 123.9 (q, 1J ...
Embodiment 2
[0068] This example provides a preparation method of 1,4-dimethoxy-2-trifluoromethyl-benzene. The structural formula of the 1,4-dimethoxy-2-trifluoromethyl-benzene is shown in the following molecular structural formula I2:
[0069]
[0070] Its preparation process is as follows:
[0071] Into a dry and clean reaction vessel, add 1mmol 1,4-dimethoxybenzene, 4mmol CF 3 SO 2 Cl, 2 mL of MeCN, 2 mmol K 2 HPO 4 , while adding 0.05mmol of ruthenium catalyst Under white light irradiation, stir for 36h.
[0072] After the reaction, the reaction solution was poured into water, extracted with ethyl acetate, and extracted three times, the organic phase was combined, dried over anhydrous sodium sulfate, and the organic phase was concentrated to obtain a crude product, which was subjected to column chromatography to obtain a purified product , the product was a white solid with a yield of 79%.
[0073] The product I2 prepared is subjected to characterization data analysis, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com